 Some BaSic conceptS of chemiStry
Some BaSic conceptS of chemiStry
According to him all substances are aggregated form of smaller units called atoms. (Paramãnu)
 SOME BASIC CONCEPTS OF CHEMISTRY
SOME BASIC CONCEPTS OF CHEMISTRY
The equation shows three carbon atoms eight hydrogen atoms
 ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES AND
ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES AND
formulas to show all the atoms including carbon and hydrogen. (a). (b). (c). (d) In this unit we have learnt some basic concepts in structure and reactivity ...
 keep501.pdf
keep501.pdf
16-Apr-2018 that all gases are at the same temperature and pressure. 29. (a) Yes. (b) ... 11 Some Basic Concepts of Chemistry. (Since density of solution ...
 Some Basic Concepts of Chemistry
Some Basic Concepts of Chemistry
Give the balanced equations for all the three half reaction. Find out the Some Basic Concepts of Chemistry 11. Page 12. 35. Moles of sugar = 34.2. 342. = 0.1.
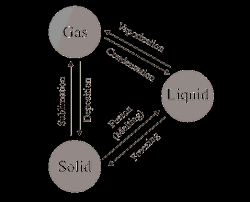 Revision Notes Class 11 Chemistry Chapter 1 - Some Basic
Revision Notes Class 11 Chemistry Chapter 1 - Some Basic
Revision Notes. Class 11 Chemistry. Chapter 1 - Some Basic Concepts of Chemistry. All the formulae of strength have amount of solute. (weight or moles) in the ...
 01. - Some Basic Concepts of Chemistry
01. - Some Basic Concepts of Chemistry
06-Apr-2020 Atoms are the. DGT Group - Tuitions (Feed Concepts) XIth – XIIth
 CHEMISTRY (Code No. 043) XI-XII (2023-24) Rationale Higher
CHEMISTRY (Code No. 043) XI-XII (2023-24) Rationale Higher
8 Organic Chemistry: Some basic Principles and. Techniques. 14. 11. 9 Hydrocarbons. 12. 10. TOTAL. 70. Unit I: Some Basic Concepts of Chemistry. 12 Periods.
 Organic chemistry – sOme Basic PrinciPles and Techniques
Organic chemistry – sOme Basic PrinciPles and Techniques
name the compounds according to IUPAC system of nomenclature and also derive their structures from the given names;. • understand the concept of organic
 CHEMISTRY (CLASSES XI –XII)
CHEMISTRY (CLASSES XI –XII)
Unit I: Some Basic Concepts of Chemistry. (Periods 14). General Introduction physical and chemical properties some important compounds: borax
 Leverage Edu
Leverage Edu
Inorganic chemistry that relates to the study of compounds of all other elements except carbon. A major focus is the study of minerals in the Earth's crust. ?
 Some Basic Concepts of Chemistry
Some Basic Concepts of Chemistry
All volumes have been reduced to NTP. Calculate the molecular formula of the hydrocarbon gas. (1979 3M) Some Basic Concepts of Chemistry 11 ...
 SOME BASIC CONCEPTS OF CHEMISTRY
SOME BASIC CONCEPTS OF CHEMISTRY
When all constituent particles of a substance are same in chemical nature it is said to be a pure substance. A mixture contains many types of particles. A
 ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES AND
ORGANIC CHEMISTRY – SOME BASIC PRINCIPLES AND
The knowledge of fundamental concepts of molecular structure helps in understanding and predicting the properties of organic compounds. You have already learnt
 SOME BASIC CONCEPTS OF CHEMISTRY
SOME BASIC CONCEPTS OF CHEMISTRY
explain various laws of chemical combination;. • appreciate significance of atomic mass average atomic mass
 D:DBTChemistry XICHEMISTRY-X
D:DBTChemistry XICHEMISTRY-X
opportunity to convey my best wishes to all the students for success in their Some Basic Concepts of Chemistry. 12. 11. Unit II Structure of Atom.
 CBSE NCERT Solutions for Class 11 Chemistry Chapter 1 - Back
CBSE NCERT Solutions for Class 11 Chemistry Chapter 1 - Back
Class-XI-CBSE-Chemistry Some Basic Concepts Of Chemistry As per stoichiometry of the balanced equation 1 mole of carbon burns in 1 mole of.
 Untitled
Untitled
CHEMISTRY SOME BASIC CONCEPTS OF CHEMISTRY www.topperlearning.com Some important applications of Chemistry are given below: o Food industry.
 CHEMISTRY (CLASSES XI –XII)
CHEMISTRY (CLASSES XI –XII)
It emphasizes a coherent focus on important ideas within the discipline that are properly sequenced Unit I: Some Basic Concepts of Chemistry.
 01. - Some Basic Concepts of Chemistry
01. - Some Basic Concepts of Chemistry
06-Apr-2020 Chemistry has played an important role in the fulfillment of basic needs of man. Explain. ... DGT MH –CET 11th CHEMISTRY Study Material.
 Some basic concept of chemistry Class 11 formula PW
Some basic concept of chemistry Class 11 formula PW
Chemistry formula for class 11 chapter- some basic concept of chemistry · Matter is substance which has mass and occupies space e g water air box table
 Some Basic Concepts Of Chemistry - Formula Sheet - Toppr
Some Basic Concepts Of Chemistry - Formula Sheet - Toppr
Get class 11 Chemistry Some Basic Concepts of Chemistry Formula Sheet here for free
 CBSE Class 11 Chemistry Formulae - StudiesToday
CBSE Class 11 Chemistry Formulae - StudiesToday
Download CBSE Class 11 Chemistry Formulae in pdf Chemistry chapter notes class notes mind maps formulas Chapter 01 Some Basic Concepts of Chemistry
 All formulas from lesson Some basic concepts of chemistry - Byjus
All formulas from lesson Some basic concepts of chemistry - Byjus
ALL FORMULAE! Q From chemistry which lesson has more weightage in the competitive exam including syllabus of 11th class
 [PDF] Some BaSic conceptS of chemiStry - NCERT
[PDF] Some BaSic conceptS of chemiStry - NCERT
Unit 1 Some BaSic conceptS of chemiStry Science can be viewed as a continuing human effort to systematise knowledge for describing and understanding
 [PDF] Some Basic Concepts of Chemistry Class 11 Leverage Edu
[PDF] Some Basic Concepts of Chemistry Class 11 Leverage Edu
Inorganic chemistry that relates to the study of compounds of all other elements except carbon A major focus is the study of minerals in the Earth's crust ?
 Some basic concept of chemistry Class 11 formula PW - Pinterest
Some basic concept of chemistry Class 11 formula PW - Pinterest
Chemistry formula for class 11 chapter- some basic concept of chemistry Chemistry This page consist of pdf sheet of science class 10th chapter-Light
 [PDF] 11_chemistry_english_2020_21pdf - Edudel
[PDF] 11_chemistry_english_2020_21pdf - Edudel
Some Basic Concepts of Chemistry 12 11 Unit II Structure of Atom 14 Unit III Classification of Elements and 08 04 Periodicity in Properties
 Notes for Chapter 1 Class 11 Chemistry – Some Basic Concepts of
Notes for Chapter 1 Class 11 Chemistry – Some Basic Concepts of
Class 11th Chemistry Chapter 1 Notes are available in PDF file on the official website of Vedantu and its app to download for free by the students Students can
What is a chemical formula for Class 11 chemistry?
The chemical formula of a compound means the symbolic representation of the composition of a compound. A chemical formula for a molecule is represented by the group of symbols of the elements that constitute the molecule, and the number of atoms of each element present in one molecule.What are some basic concepts of chemistry for class 11th?
Every substance has unique or characteristic properties. These properties can be classified into two categories — physical properties, such as colour, odour, melting point, boiling point, density, etc., and chemical properties, like composition, combustibility, ractivity with acids and bases, etc.What are the basic formulas in chemistry?
There are three main types of chemical formulas: empirical, molecular and structural.
Page 1 of 29
Practice more www.embibe.com
CBSE NCERT Solutions for Class 11 Chemistry Chapter 1Back from chapter Questions
1. Calculate the molar mass of the following:
(iii) ସsSolution:
oxygen) ൌ(ͳൈ Atomic mass of carbon)(ʹൈ Atomic mass of oxygen) (iii) The molecular mass of methane ( ସ) Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 2 of 29
Practice more www.embibe.com
Solution:
Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 3 of 29
Practice more www.embibe.com
dioxygen by mass.Solution:
Relative moles of oxygen in iron oxide:
Simplest molar ratio of iron to oxygen:
4. Calculate the amount of carbon dioxide that could be produced when
(i) ͳ mole of carbon is burnt in air. (ii) ͳ mole of carbon is burnt in ͳ of dioxygen. (iii) ʹ moles of carbon is burnt in ͳ of dioxygen. Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 4 of 29
Practice more www.embibe.com
Solution:
The balanced reaction of combustion of carbon can be written as: Initial mole 1 1 െ Left mole ͳെͳൌͲ ͳെͳൌͲ 1 As per stoichiometry of the balanced equation, ͳ mole of carbon burns in ͳ mole of dioxygen (air) to Produced ͳ mole of carbon dioxide. (ii) ͳ mole of carbon is burnt in ͳ of dioxygen. Given in question : dioxygen= ͳ ; mole of carbon = 1We know : ൌ୫୭୪ୣୱ
According to the stoichiometry of the balanced equation (ʹʹ). Hence, dioxygen is a limiting reactant. Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 5 of 29
Practice more www.embibe.com
Solution:
Volume of solution = 500 ml
solution (litre)6. Calculate the concentration of nitric acid in moles per liter in a sample which has a
Solution:
Mass percent of nitric acid in the sample ൌͻΨ Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 6 of 29
Practice more www.embibe.com
We know, the mass percent of nitric acid in it being ͻΨൌ ͳͲͲ of nitric acid contains
ͻ of nitric acid by mass.
Concentration of nitric acidൌ୫୭୪ୣSolution:
Given : mass of ସൌ 100 g
Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 7 of 29
Practice more www.embibe.com
8. Determine the molecular formula of an oxide of iron, in which the mass percent of iron
Solution:
We know : The atomic mass of iron = 55.85
The atomic mass of oxygen = 16
The ratio of iron to oxygen in the oxide,
ൌͳ(approx) Molecular formula of a compound = (empirical formula of a compound) . Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 8 of 29
Practice more www.embibe.com
9. Calculate the atomic mass (average) of chlorine using the following data:
Ψ Natural
Abundance
Molar Mass
Solution:
given in question :The average atomic mass of chlorine.
(i) Number of moles of carbon atoms. (ii) Number of moles of hydrogen atoms. (iii) Number of molecules of ethane.Solution:
Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 9 of 29
Practice more www.embibe.com
ൌʹൈ͵ൌ moles of carbon ൌ͵ൈൌͳͺ moles of hydrogen atoms ethane. enough water to make a final volume up to ʹ?Solution:
Given mass of glucose = 20 g
By putting the values in formula we get
Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 10 of 29
Practice more www.embibe.com
Solution:
Given:
13. Pressure is determined as force per unit area of the surface. The unit of pressure, pascal
is as shown below:Solution:
The pressure is defined as force acting per unit area of the surface. Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 11 of 29
Practice more www.embibe.com
We know,
Then,14. What is the unit of mass? How is it defined?
Solution:
ͳ kilogram is defined as the mass equal to the mass of the international prototype(Platinum-Iridium cylinder in Paris) of the kilogram.15. Match the following prefixes with their multiples:
Prefixes Multiples
(i) Micro ͳͲ (ii) Deca ͳͲଽ (iii) Mega ͳͲି (iv) Giga ͳͲିଵହ (v) Femto ͳͲSolution:
Prefixes Multiples
(i) Micro ͳͲି (ii) Deca ͳͲ (iii) Mega ͳͲ (iv) Giga ͳͲଽ (v) Femto ͳͲିଵହ Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 12 of 29
Practice more www.embibe.com
16. What do you mean by significant figures?
Solution:
Significant figures are those meaningful digits that are known with certainty. They result of an experiment, then ʹͷ is certain, while ͺ is uncertain, and the total number of significant figures are ͵. Hence, significant figures are defined as the total number of digits in a number including the last digit that represents the uncertainty of the result.17. A sample of drinking water was found to be severely contaminated with chloroform
ଷ supposed to be carcinogenic in nature. The level of contamination was ͳͷ ppm (by mass). (i) Express this in percent by mass. (ii) Determine the molality of chloroform in the water sample.Solution:
Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 13 of 29
Practice more www.embibe.com
Formula : Molaity =
Put all the above values in formula we get
Molality of chloroform in water.
18. Express the following in the scientific notation:
(iii) ͺͲͲͺSolution:
19. How many significant figures are present in the following?
(ii) ʹͲͺ (iii) ͷͲͲͷ Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 14 of 29
Practice more www.embibe.com
(iv) ͳʹǡͲͲͲSolution:
(i) There are ʹ significant figures. (ii) There are ͵ significant figures. (vi) There are ͵ significant figures. (vi) There are ͷ significant figures.20. Round up the following upto three significant figures:
(iv) ʹͺͲͺSolution:
(iv) ʹͺͳͲ21. The following data are obtained when dinitrogen and dioxygen react together to form
different compounds:Mass of dinitrogen Mass of dioxygen
(iii) ʹͺ ͵ʹ (iv) ʹͺ ͺͲ Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 15 of 29
Practice more www.embibe.com
(a) Which law of chemical combination is obeyed by the above experimental data? Give its statement. (b) Fill in the blanks in the following conversions: (i) ͳൌ ____________ ൌ ____________ (ii) ͳൌ ____________ ൌ ____________ (iii) ͳൌ ____________ ൌ ____________ ଷSolution:
(a) ʹͺ Mass of dinitrogen Mass of dioxygen Ration of oxygen (iii) ʹͺ ͵ʹ 32 (iv) ʹͺ 80 (b) (i) ͳൌͳൈଵ୫ Hence, ͳൌͳͲൌͳͲଵହ (ii) ͳൌͳൈଵ Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 16 of 29
Practice more www.embibe.com
(iii) ͳൌͳൈଵSolution:
distance covered by light =?Formula : Distance traveled by light ൌൈ
Identify the limiting reagent, if any, in the following reaction mixtures. (i) ͵ͲͲ atoms of ʹͲͲ molecules of (ii) ʹ͵ (iii) ͳͲͲ atoms of ͳͲͲ molecules of Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 17 of 29
Practice more www.embibe.com
Solution:
A reagent determines the extent of a reaction. It is the reactant which is the first to get consumed during a reaction, thereby causing the reaction to stop and limiting the amount of products formed. (i) ͵ͲͲ atoms of ʹͲͲ molecules of According to the stoichiometry of reaction, ͳ atom of reacts with ͳ molecule of . Initial moles ͵ͲͲൌ͵ͲͲ 200 molecule =ʹͲͲ Left mole (300-200) =100 0 ʹͲͲ (ii) ʹ͵ According to the reaction, ͳ of reacts with ͳ of ͵. Initial moles 2 3 െ ଵ=2 ଷLeft mole 2-2=0 3-2=1 2
Thus, ʹ of will react with only ʹ of . As a result, ͳ of will not be consumed. Hence, is the limiting reagent. (iii) ͳͲͲ atoms of ͳͲͲ molecules of According to the given reaction, ͳ atom of combines with ͳ molecule of . Initial moles ͳͲͲൌͳͲͲ 100 molecule =ͳͲͲ ଵ=100 ଵఽ Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 18 of 29
Practice more www.embibe.com
Left mole 0 0 ͳͲͲ
Thus, all 100 moles of will combine with all 100 moles of . Hence, the mixture is stoichiometric where no limiting reagent is present. limiting reagent. According to the reaction, ͳ of atom combines with ͳ of molecule . Thus, left as such. Hence, is the limiting reagent.24. Dinitrogen and dihydrogen react with each other to produce ammonia according to the
following chemical reaction: (ii) Will any of the two reactants remain unreacted? (iii) If yes, which one and what would be its mass? Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 19 of 29
Practice more www.embibe.com
Solution:
we should know : ݈݉݁ܿݑ݈ܽBalancing the given chemical equation,
From the equation, ͳ mol of nitrogen reacts with ͵ mol of dihydrogen to give ʹ moles of ammonia. unreacted. Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 20 of 29
Practice more www.embibe.com
Solution:
26. If ͳͲ volumes of dihydrogen gas reacts with five volumes of dioxygen gas, how many
volumes of water vapour would be produced?Solution:
Reaction of dihydrogen with dioxygen can be written as:Mole ratio 2 1 2 moles ן
According to stiochimerty of reaction two volumes of dihydrogen react with one volume of dioxygen to produce two volumes of water vapour. Hence, ten volumes of dihydrogen will react with five volumes of dioxygen to produce ten volumes of water vapour.27. Convert the following into basic units:
(iii) ʹͷ͵ͷ Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 21 of 29
Practice more www.embibe.com
Solution:
(iii) ʹͷ͵ͷ:Since,
28. Which one of the following will have the largest number of atoms?
Solution:
Atomic mass of Au = 107
Atomic mass of Na = 23
Atomic mass of Li = 7
Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 22 of 29
Practice more www.embibe.com
29. Calculate the molarity of a solution of ethanol in water, in which the mole fraction of
Solution:
We know : density of water = 1 gm/ml
Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 23 of 29
Practice more www.embibe.com
Solution:
ൌͳʹ of carbon Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 24 of 29
Practice more www.embibe.com
31. How many significant figures should be present in the answer of the following
calculations?Solution:
ൌ Number of significant figures in the least precise number. Since the least number of decimal places in each term is four, the number of significant figures in the answer is also 4.32. Use the data given in the following table to calculate the molar mass of naturally
occuring argon isotopes:Isotope Isotopic molar mass Abundance
Class-XI-CBSE-Chemistry Some Basic Concepts Of ChemistryPage 25 of 29
Practice more www.embibe.com
Solution:
Given :
for Isotope ଷ for Isotope ଷ଼ for IsotopeAverage molar mass
Average molar mass of argon
33. Calculate the number of atoms in each of the following
quotesdbs_dbs22.pdfusesText_28[PDF] somme de suite arithmétique
[PDF] somme de suite arithmétique formule
[PDF] somme latex
[PDF] son architecture
[PDF] son citation machine apa 6th edition
[PDF] son of citation machine apa book
[PDF] son of citation machine apa journal
[PDF] sonali bank dollar rate today
[PDF] sonata recalls
[PDF] songs with piano chords pdf
[PDF] sonia futures
[PDF] sonic cisco tx
[PDF] sonicwall cisco site to site vpn
[PDF] sonicwall cisco vlan trunk
