 High Purity Ethyl Acetate Production with a Batch Reactive
High Purity Ethyl Acetate Production with a Batch Reactive
These works have shown the difficulty to achieve the industrial grade ethyl acetate in a shift of 8 hrs. control problem which is formulated to determine an ...
 Specification - Pearson BTEC Level 3 National Extended Diploma in
Specification - Pearson BTEC Level 3 National Extended Diploma in
investigate the different methods for testing the purity of the products. o industrial scale – from ethanol and ethanoic acid (for ethyl ethanoate) o ...
 8-Synthesis-of-Aspirin.pdf
8-Synthesis-of-Aspirin.pdf
The collected aspirin will be tested for its purity using FeCl3 (aq). Iron (III) ion reacts with phenols to form a purple complex. Salicylic acid contains a
 GENERAL TESTS PROCESSES AND APPARATUS
GENERAL TESTS PROCESSES AND APPARATUS
Purity of crude drug (17:3) at a rate of not more than 5 mL per minute ... ethyl acetate and water (4:4:3) as the developing solvent: the standard solution ...
 UK Standard Industrial Classification of Economic Activities 2007
UK Standard Industrial Classification of Economic Activities 2007
This group includes the manufacture of measuring testing and navigating equipment for various industrial and non-industrial testing of composition and purity ...
 Aspirin
Aspirin
to test for purity. ... s Access to a few iodine crystals (three or four per experiment is enough) s Samples for testing s Ethyl ethanoate as chromatography ...
 Ethanol to Ethyl Acetate
Ethanol to Ethyl Acetate
20 May 2019 The ethyl acetate produced has a weight purity of 99.8% meeting market specifications. This process makes use of three main unit operations
 Student Safety Sheets
Student Safety Sheets
testing. Limited amounts of Red Bull or similar
 Practical handbook
Practical handbook
To test the purity of an organic solid by measuring its melting point t) As the ethyl ethanoate vapours start to carry over and condense record the ...
 Investigating Esters
Investigating Esters
Plan to prepare a sample of butyl ethanoate ethyl ethanoate or methyl ethanoate. Unknown author
 Integration of the Process for Production of Ethyl Acetate by an
Integration of the Process for Production of Ethyl Acetate by an
17 Aug 2021 Ethyl acetate (EA) in the chemical industry is obtained mainly by the ... This is a particular problem when high purity ethyl acetate is.
 High Purity Ethyl Acetate Production with a Batch Reactive
High Purity Ethyl Acetate Production with a Batch Reactive
22 Oct 2010 on the industrial grade ethyl acetate production operating in a batch mode. ... control problem which is formulated to determine an optimal.
 BTEC Assignment Brief
BTEC Assignment Brief
B: Explore the manufacturing techniques and testing methods for an organic liquid Prepare a sample of ethyl ethanoate and test its purity.
 Q3C (R6) Step 5 - impurities: guideline for residual solvents
Q3C (R6) Step 5 - impurities: guideline for residual solvents
9 Aug 2019 of the solvent for the synthesis of drug substance may enhance the yield or determine characteristics such as crystal form
 Aspirin
Aspirin
Ethyl ethanoate is highly flammable and the vapour may irritate the eyes and Chromatography techniques are used a great deal in industry because they ...
 Higher Nationals
Higher Nationals
paracetamol antifebrin; a liquid organic compound eg ethyl ethanoate
 GENERAL TESTS PROCESSES AND APPARATUS
GENERAL TESTS PROCESSES AND APPARATUS
microscopic examination purity test
 8-Synthesis-of-Aspirin.pdf
8-Synthesis-of-Aspirin.pdf
The collected aspirin will be tested for its purity using FeCl3 (aq). Iron (III) ion reacts with phenols to form a purple complex. Salicylic acid contains a
 Aspirin
Aspirin
Ethyl ethanoate is volatile highly flammable and the vapour may irritate the eyes and respiratory system. Chemical tests for purity.
 rification_and_testing_of_ethyl_ethanoate_in_the_laboratorydocx
rification_and_testing_of_ethyl_ethanoate_in_the_laboratorydocx
High Purity Ethyl Acetate Production with a Batch Reactive Distillation Column using Dynamic Optimization Strategy Abstract—Ethyl acetate with the minimum purity of 85 0 by mole is inevitably needed as an active solvent used in a wide range of applications across many industries
Is industrial manufacture of ethyl ethanoate preferred method of production?
- The gas produced should not be smelled directly instead be wafted to avoid interaction with the respiratory system. Conclusion and evaluations In conclusion, industrial manufacture of ethyl ethanoate is more preferred method of production, as large amount of output of the products is obtained.
What are ethyl ethanoate & ethanol ester?
Esters can be made from carboxylic acids and alcohols. This is discussed in detail on another page; in general terms, the two combine together, losing a molecule of water in the process. Consider a very simple ester such as ethyl ethanoate. The figure below shows its formation from ethanoic acid and ethanol.
What is the maximum purity of ethyl acetate?
It is normally produced by the esterification of ethanol and acetic acid. Due to the equilibrium limitation, it has been reported that the ethyl acetate with the maximum purity of 52.0% can be obtained in a batch reactor. To achieve higher purity, further purification unit is added with the use of some energy.
What does ethylethanoate smell like?
Abundance acid and alcohol bothdissolve and are tucked securely away under the ester layer. Little esters like ethylethanoate smell like ordinary natural solvents (ethyl ethanoate is a common solventin, for example, glues). As the esters get greater, the smells tend towards artificialfruit flavouring - "pear drops", for example.
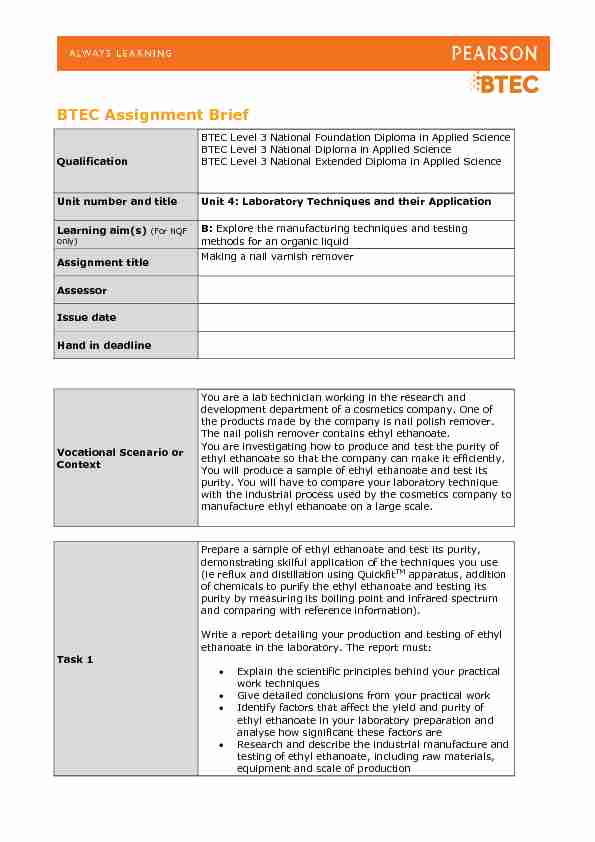
BTEC Assignment Brief
Qualification
BTEC Level 3 National Foundation Diploma in Applied ScienceBTEC Level 3 National Diploma in Applied Science
BTEC Level 3 National Extended Diploma in Applied ScienceUnit number and title
Unit 4: Laboratory Techniques and their ApplicationLearning aim(s) (For NQF
only) B: Explore the manufacturing techniques and testing methods for an organic liquidAssignment title Making a nail varnish remover
Assessor
Issue date
Hand in deadline
Vocational Scenario or
Context
You are a lab technician working in the research and development department of a cosmetics company. One of the products made by the company is nail polish remover.The nail polish remover contains ethyl ethanoate.
You are investigating how to produce and test the purity of ethyl ethanoate so that the company can make it efficiently. You will produce a sample of ethyl ethanoate and test its purity. You will have to compare your laboratory technique with the industrial process used by the cosmetics company to manufacture ethyl ethanoate on a large scale.Task 1
Prepare a sample of ethyl ethanoate and test its purity, demonstrating skilful application of the techniques you use (ie reflux and distillation using QuickfitTM apparatus, addition of chemicals to purify the ethyl ethanoate and testing its purity by measuring its boiling point and infrared spectrum and comparing with reference information). Write a report detailing your production and testing of ethyl ethanoate in the laboratory. The report must: Explain the scientific principles behind your practical work techniques Give detailed conclusions from your practical work Identify factors that affect the yield and purity of ethyl ethanoate in your laboratory preparation and analyse how significant these factors are Research and describe the industrial manufacture and testing of ethyl ethanoate, including raw materials, equipment and scale of production 2BTEC Assignment Brief v1.0
BTEC Internal Assessment QDAM January 2015
Compare the laboratory and industrial manufacture
and testing of ethyl ethanoate, explaining principles, similarities and differences in the equipment and techniques used Analyse how relevant the factors you have identified as affecting yield and purity are in the industrial manufacture of ethyl ethanoateAnalyse whether boiling point measurement and
infrared spectroscopy are effective ways to assess purity of a liquid, and draw a conclusion on whether other methods used industrially, such as chromatography, are more reliable.Checklist of evidence
required A report on the practical work and the detailed conclusions from this, including the analysis of factors affecting yield and purity, and research on industrial manufacture and testing An observation report by the teacher of making and testing the liquid safely.All information sources should be referenced.
Criteria covered by this task:
Unit/Criteria
reference To achieve the criteria you must show that you are able to: B.D2 Analyse the factors affecting the yield and purity of an organic liquid in the laboratory and their relevance to its industrial manufacture. B.M2 Demonstrate skilful application of techniques in preparing and testing the purity of an organic liquid and draw detailed conclusions. B.M3 Compare the laboratory and industrial manufacture and testing of an organic liquid. B.P3 Prepare and test the purity of an organic liquid and draw conclusions. B.P4 Describe the industrial manufacture and testing of an organic liquid.Other assessment
materials attached to this Assignment Briefquotesdbs_dbs7.pdfusesText_5[PDF] tex's french grammar prepositions with places answers
[PDF] tex's french grammar pronominal verbs answers
[PDF] tex's french grammar pronoun en answers
[PDF] tex's french grammar pronoun y answers
[PDF] tex's french grammar questions with subject/verb inversion answers
[PDF] texas ant identification chart
[PDF] texas area code map
[PDF] texas attorney general child support chart 2019
[PDF] texas blue 2024
[PDF] texas bureau of prisons
[PDF] texas carpenter ants
[PDF] texas certificate of conversion
[PDF] texas climate
[PDF] texas code of criminal procedure chapter 15
