 Determine the rate constant of the saponification of Ethylacetate by
Determine the rate constant of the saponification of Ethylacetate by
A) Requirements: Ethylacetate solution (N/25) NaOH (N/25)
 Determination of the Expression Rate of Ethyl Acetate Hydrolysis
Determination of the Expression Rate of Ethyl Acetate Hydrolysis
Kinetic studies on saponification of ethyl acetate using an Reaction rate and rate constant of the hydrolysis of ethyl acetate with sodium hydroxide.
 TO STUDY THE KINETICS OF SAPONIFICATION OF AN ESTER BY
TO STUDY THE KINETICS OF SAPONIFICATION OF AN ESTER BY
You would perform the reaction using equal concentrations of the ethyl acetate and sodium hydroxide. Here again the progress of the reaction will be followed.
 Jawaharlal Nehru Engineering College
Jawaharlal Nehru Engineering College
A] Aim: To determine the reaction rate constant (K) for saponification of ethyl acetate with sodium hydroxide at ambient conditions. Chemicals required:.
 CHEMICAL REACTION ENGINEERING LABORATORY LAB MANUAL
CHEMICAL REACTION ENGINEERING LABORATORY LAB MANUAL
NaOH at ambient conditions. 6. To determine the reaction rate constant for saponification of ethyl acetate with. NaOH at a fixed temp. To study the effect of
 Chemical Kinetics and Reaction Engineering (CHC304)
Chemical Kinetics and Reaction Engineering (CHC304)
AIM: To determine the rate constant for saponification reaction of ethyl acetate and sodium hydroxide in a straight tube type plug flow reactor at ambient
 Reaction Engg. & Thermodynamics Lab
Reaction Engg. & Thermodynamics Lab
Determine the reaction rate constant for the saponification of ethyl acetate with sodium hydroxide. 5. Determine the partial molar volume of a binary liquid
 Kinetic studies on saponification of ethyl acetate using an innovative
Kinetic studies on saponification of ethyl acetate using an innovative
tively to determine rate constant of saponification of ester. However fication reaction of ethyl acetate and sodium hydroxide. [4
 Experimental Investigations on Shifting Order Behavior of Ethyl
Experimental Investigations on Shifting Order Behavior of Ethyl
30-Jan-2020 that saponification of ethyl acetate with sodium hydroxide does ... “Reaction Rate and Rate Constant of the Hydrolysis of Ethyl Acetate with ...
 Reaction rate and rate constant of the hydrolysis of ethyl acetate with
Reaction rate and rate constant of the hydrolysis of ethyl acetate with
In this hydrolysis of ester (ethyl acetate) with an alkali (sodium hydroxide) HCl was from the reaction mixture and determining the NaOH present in it ...
 Saponification of Ethyl Acetate
Saponification of Ethyl Acetate
k = specific reaction rate. The bimolecular reaction studied in this experiment is the saponi- fication of an ester by sodium hydroxide.
 Determine the rate constant of the saponification of Ethylacetate by
Determine the rate constant of the saponification of Ethylacetate by
For determination of C0 only NaOH solution of same concentration need to be measured. C) Procedure: 1. Preparation of exact N/10 Oxalic acid standard solution.
 Determination of the Expression Rate of Ethyl Acetate Hydrolysis
Determination of the Expression Rate of Ethyl Acetate Hydrolysis
the reaction order the forward rate constant
 Experimental Studies of Ethyl Acetate Saponification Using Different
Experimental Studies of Ethyl Acetate Saponification Using Different
4 févr. 2019 CH COOC H + NaOH ? CH COONa + C H OH. (2) where k denotes the reaction rate constant. The conversion of sodium hydroxide was calculated:.
 Reaction rate and rate constant of the hydrolysis of ethyl acetate with
Reaction rate and rate constant of the hydrolysis of ethyl acetate with
Chemical kinetics is the part of physical chemistry that studies reaction rates. The reaction rate for a reactant or product in a particular reaction is.
 Demulsification of a Mixture of Di-Chloro-Floro-Acetophenone Di
Demulsification of a Mixture of Di-Chloro-Floro-Acetophenone Di
1 févr. 2017 Keywords: Hydrolysis; Reaction Kinetics; Order of Reaction; Rate ... Saponification of ethyl acetate with sodium hydroxide.
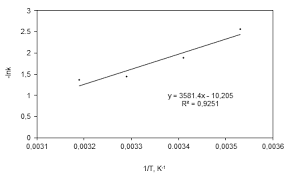
 Estimation of Parameters of Kinetic Study and Arrhenius Equation
Estimation of Parameters of Kinetic Study and Arrhenius Equation
19 mars 2019 the Reaction between Ethyl Acetate with Sodium Hydroxide ... reaction activation energy
 CHEMICAL REACTION ENGINEERING LABORATORY LAB MANUAL
CHEMICAL REACTION ENGINEERING LABORATORY LAB MANUAL
NaOH at ambient conditions. 6. To determine the reaction rate constant for saponification of ethyl acetate with. NaOH at a fixed temp. To study the effect
 Estimation of Parameters of Arrhenius Equation for Ethyl Acetate
Estimation of Parameters of Arrhenius Equation for Ethyl Acetate
16 nov. 2015 constant and activation energy for ethyl acetate saponification. ... Previous study shows that Specific rate constant and conversion.
 REACTION ENGINEERING 5.1. Determination of Kinetic Parameters
REACTION ENGINEERING 5.1. Determination of Kinetic Parameters
Sodium Hydroxide Mol wt.: 40.00 (pellet). 5.1.1. Aim. To study the saponification of ethyl acetate in a plug-flow reactor and to determine the kinetic.
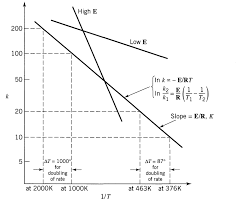
 Experiment C5 Chemistry 114 Kinetics in Solution
Experiment C5 Chemistry 114 Kinetics in Solution
Goals: To measure rate constants for the saponification of Ethyl acetate in sodium hydroxide solutions at several temperatures From these rate constants the activation energy and entropy of activation can be calculated Additional experiments will explore the order of the reaction and the saponification rate of other esters Background
What is the saponification reaction between sodium hydroxide (NaOH) and ethyl acetate (et?
Introduction: In this reaction, the saponification reaction between sodium hydroxide (NaOH) and ethyl acetate (EtAc) was conducted. The reaction was conducted using equimolar amounts of both reactants to produce ethanol (EtOH) and sodium acetate (NaAc). In addition to, the kinetic parameters for the reaction were determined.
What equimolar reactants were used in a saponification reaction?
This was a saponification reaction. The reaction was carried using equimolar reactants that yielded sodium acetate and ethanol. The safety data for the materials used in this reaction can be found in Appendix F. The purpose of this experiment was to study how different reactors undergo the same reaction, analyzing and comparing them to each other.
What is a second order reaction between sodium hydroxide and ethyl acetate?
The addressed system is characterized by an endothermic second-order reaction between sodium hydroxide (NaOH) and ethyl acetate (CH 3 COOCH 2 CH 3 ), as described in Equation (15). This reaction is carried out in a 1.2L stirred batch reactor and follows the kinetic parameters according to .
How does agitation rate affect the conversion of sodium hydroxide?
The conversion of sodium hydroxide declines with increased agitation rate from 70 rpm to 150 rpm. Higher initial reactant concentration leads to decreased reaction conversion. The results obtained may be used to optimize the production of ethanol and sodium acetate using saponification reaction.
[PDF] the success of 7 eleven japan pdf
[PDF] the suitors in the odyssey
[PDF] the swift programming language (swift 5.0) pdf download
[PDF] the swift programming language (swift 5.1) pdf
[PDF] the swift programming language (swift 5.1) pdf download
[PDF] the swift programming language (swift 5.2) pdf download
[PDF] the swift programming language 4.2 pdf download
[PDF] the talent code book in hindi pdf
[PDF] the ten commandments
[PDF] the ten commandments book pdf
[PDF] the ten commandments explained pdf
[PDF] the ten commandments in order
[PDF] the tenth amendment of the bill of rights
[PDF] the third level class 12 important questions
