 Dalal Institute
Dalal Institute
Join the revolution by becoming a part of our community and get all of the member benefits like downloading any PDF document for your personal preview. Sign
 Thermodynamics Properties of Pure Substance
Thermodynamics Properties of Pure Substance
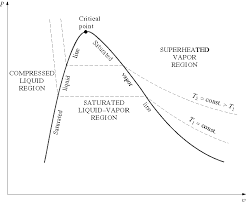
A substance is said to be superheated if the given temperature is greater than the saturation temperature for the given pressure. State 5 in Figure 2-3 (page 3)
 ENGINEERING THERMODYNAMICS
ENGINEERING THERMODYNAMICS
2 : Reversibe adiabatic process : p. 1. V. 1 γ = p. 2. V. 2 γ. V. V p p. 2. 1. 1. 2. 1. = F. HG. I. KJ γ. Page 160. FIRST LAW OF THERMODYNAMICS. 137 dharm. /M- ...
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
há 8 dias ... pdf. 2W. Kaplan 2003
 The two parts of the second law of thermodynamics
The two parts of the second law of thermodynamics
9 de jul. de 2018 That is the reason why in the processes (2) and (4) of the cycle the working agent is in isolation meaning that there is no exchange of heat.
 Thermodynamics Tables and Charts
Thermodynamics Tables and Charts
International Standard Formulation for the Thermodynamic Properties of 11
 UNIT – I – Thermodynamics-II – SCY1316
UNIT – I – Thermodynamics-II – SCY1316
UNIT – I – Thermodynamics-II – SCY1316. Page 2. 2. 1. INTRODUCTION. Need of Second Law of Thermodynamics. 1. First law states that “heat can be converted
 Fundamentals of Engineering Thermodynamics
Fundamentals of Engineering Thermodynamics
thermodynamics also deals with phenomena not included within the scope of mechanics ... 2 denote the inlet and exit respectively
 2 Thermodynamic Property Models
2 Thermodynamic Property Models
Aspen Physical Property System thermodynamic property models include classical thermodynamic property models such as activity coefficient models and equations
 Thermodynamics And An Introduction To Thermostatistics-Wiley
Thermodynamics And An Introduction To Thermostatistics-Wiley
Thermodynamics 1960. Bibliography p 485. Includes index. 1 Thermodynamics. I Callen Herbert B. 2 Statistical Mechanics. Thermodynamics II Title. III Title
 Chemical Engineering Thermodynamics II
Chemical Engineering Thermodynamics II
Chapter 2: Thermodynamic Property Relationships. 2.1. Type of Thermodynamic Properties. 2-1 4http://students.aiche.org/pdfs/thermodynamics.pdf 11/27/04.
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
Feb 14 2010 2.4.2 Non-ideal thermal equations of state . ... These are lecture notes for AME 20231
 ENGINEERING THERMODYNAMICS
ENGINEERING THERMODYNAMICS
Zeroth Law of Thermodynamics 23. 2.15. The Thermometer and Thermometric Property ... 24. 2.15.1. Introduction ... 24. 2.15.2. Measurement of temperature.
 Basic Concepts of Thermodynamics Thermodynamics and Energy
Basic Concepts of Thermodynamics Thermodynamics and Energy
velocity (m/s2) pressure (Pa = kg/m.s2). There are two unit systems currently available SI (International System) and USCS (United.
 The Second Law of Thermodynamics
The Second Law of Thermodynamics
2: Steam power plant is a heat engine. Thermal efficiency: is the fraction of the heat input that is converted to the net work output (efficiency = benefit /
 PDF First Law of Thermodynamics Control Volumes
PDF First Law of Thermodynamics Control Volumes
2: Energy content of CV can be changed by mass flow in/out and heat and work interactions. Work flow: is the energy that is required to push fluid into or out
 Introduction to chemical engineering thermodynamics
Introduction to chemical engineering thermodynamics
%20Hendrick%20Van%20Ness
 1 General Chemistry II Jasperse Entropy Spontaneity
1 General Chemistry II Jasperse Entropy Spontaneity
http://web.mnstate.edu/jasperse/Chem210/Extra%20Practice%20Sets%20Chem%20210/Test3ch14-Thermo-Practice.pdf
 Chapter 3 Thermodynamic Properties
Chapter 3 Thermodynamic Properties
48. Page 2. 3-2. Pressure can be expressed as a function of temperature and specific volume
 Thermodynamic Properties and calculation
Thermodynamic Properties and calculation
BASIC CONCEPTS-2. ? PV diagram. ? Virial Equations of State. PV = a + bP + cP2 + ... ?. ? Ideal gas: Z=1 or PV = RT.
 Lecture 5: Thermodynamics - Scholars at Harvard
Lecture 5: Thermodynamics - Scholars at Harvard
Lecture5: Thermodynamics 1 Introduction Thermodynamicsis thestudyof heat andtemperature Onethingthat makes thermodynamicshard(andgenerallyunpopular) is all the damnvariables Everything is relatedandit's oftentoughtokeepstraight whatisanindependentandwhat is adependent variable
 Thermodynamics – II - Dalal Institute
Thermodynamics – II - Dalal Institute
Thermodynamics – II Clausius-Clapeyron Equation The Clausius-Clapeyron equation was initially proposed by a German physics Rudolf Clausius in 1834 and then further developed by French physicist Benoît Clapeyron in 1850 This equation is extremely
 Thermodynamics PDF: Definitions Basics Statements Laws and - BYJ
Thermodynamics PDF: Definitions Basics Statements Laws and - BYJ
ME 312– Thermodynamics II (Required) Catalog Description: ME 312 (3 0 3) continuation of ME 311 including studies of irreversibility and combustion Thermodynamic principles are applied to the analysis of power generation refrigeration and air-conditioning systems
 THERMODYNAMICS: COURSE INTRODUCTION
THERMODYNAMICS: COURSE INTRODUCTION
The thermodynamic state of a system is defined by specifying a setof measurable properties sufficient so that all remainingproperties are determined Examples of properties: pressuretemperature density internal energy enthalpy and entropy
What are the laws of thermodynamics?
Laws of thermodynamics gives a clear insight about energy, entropy, and thermal equilibrium of any system. The first law of thermodynamics, which is also known as the Law of Conservation of Energy, states that energy can neither be created nor be destroyed, it can only be transferred from one form to another.
What are the different types of thermodynamics?
Thermodynamic systems can be classified as follows: Open: Those systems that exchange matter and energy with the outside. Closed: Are those that exchange only energy with the outside. Isolated: Those that do not exchange neither energy nor matter.
What is the zeroth law of thermodynamics?
The Zeroth Law of thermodynamics states that there is an energy form called heat, which has the tendency to spread through a system, and a variable called temperature that measures this tendency: heat flows from the regions of high temperature to the regions of low temperature only. What is entropy law?
What are the applications of thermodynamics?
The understanding of thermodynamic phenomena is very useful in the fields of engineering, architecture, chemistry and biology. Especially, where enormous amounts of energy are needed to start up various machines. Also, the laws of thermodynamics help a lot in some disciplines such as genetics.
ON THE CD (Software and Simulations)
QUICKFIELD
STUDENTS' VERSION (v. 5.6)
QuickField is a Finite Element Analysis package for elec- tromagnetic, thermal, and stress design simulation with coupled multi-Þ eld analysis. Also includes tutorials.By Tera Analysis Ltd.
http://www.quickÞ eld.comCALCULATION OF HIGH-PRESSURE CHEMICAL
EQUILIBRIUM: CASE OF AMMONIA SYNTHESIS
By Housam Binous, PhD
http://www.mathworks.com/matlabcentral/ Þ leexchange/17829THERMODYNAMIC PROPERTIES OF WATER
By François Brissette, PhD
ENGINEERING
THERMODYNAMICS
THIRD EDITION
SI Units Version
R. K. Rajput
Intended as an introductory textbook for "applied" or engineering thermodynamics, or for use as an up-to-date reference for practicing engineers, this book provides extensive in-text, solved examples to cover the basic properties of thermodynamics. Pure substances, the rst and second laws, gases, psychrometrics, the vapor, gas, and refrigeration cycles, heat transfer, compressible ow, chemical reactions, fuels, and more are presented in detail and enhanced with practical applications. This version presents the material using SI Units and has ample material on SI conversion, steam tables, and a Mollier diagram. The accompanying CD includes a fully func- tional student version ofQuickField
software (widely used in industry) with simulations, tutorials, etc.KEY FEATURES
Uses extensive, in-text, solved examples
(with computer simulations on the CD) to cover the basic properties of engineering thermodynamics and heat transferPresents the material using SI Units and
has ample material on SI conversion, steam tables, and a Mollier diagramIncludes a CD-ROM with QuickField soft-
ware, MATLAB simulations, and guresABOUT THE AUTHOR
R. K. Rajput
has over 35 years of experience teaching mechanical and electrical engineering and ha s authoredseveral books and journal articles in these areas. He has won many distinguished awards for both teaching
and research. ENGINEERING
THERMODYNAMICS
THIRD EDITION
ENGINEERING
THERMODYNAMICS
THIRD EDITION
SI Units Version
ENGINEERING SERIESRAJPUT
R. K. Rajput
All trademarks and service marks are the property of their respective ow ners.Jones and Bartlett Publishers
40 Tall Pine Drive
Sudbury, MA 01776
978-443-5000
info@jbpub.com www.jbpub.comJones andBartlettrajput_thermodynamics.indd 1rajput_thermodynamics.indd 12/5/09 12:56:11 PM2/5/09 12:56:11 PM
ENGINEERING THERMODYNAMICS
DHARMM-therm\TITLE.PM5 i i
Also available:STEAM TABLES
andMOLLIER DIAGRAM
(S.I. UNITS)Edited
byR.K. RAJPUT
Patiala
BANGALORECHENNAICOCHINGUWAHATIHYDERABAD
JALANDHAR
KOLKATALUCKNOWMUMBAIRANCHI
NEW DELHIBOSTON, USA
ENGINEERING
THERMODYNAMICS
[For Engineering Students of All Indian Universities and Competitive Examinations]S.I. UNITS
ByR.K. RAJPUT
M.E. (Heat Power Engg.) Hons.-Gold Medallist ; Grad. (Mech. Engg. & Elect. Engg.) ; M.I.E. (India) ; M.S.E.S.I. ; M.I.S.T.E. ; C.E. (India)Principal (Formerly)
Punjab College of Information Technology
PATIALA, Punjab
Published by :
LAXMI PUBLICATIONS (P) LTD
113, Golden House, Daryaganj,
New Delhi-110002
Phone : 011-43 53 25 00
Fax : 011-43 53 25 28
www.laxmipublications.com info@laxmipublications.com© All rights reserved with the Publishers.
No part of this publication may be reproduced, stored in a retrieval sys tem, or transmitted in any form or by any means, electronic, mechanical, photocopying, recording or otherwise without the prior written permission of the publi sher.ISBN: 978-0-7637-8272-6
3678Price : Rs. 350.00 Only.First Edition : 1996
Second Edition : 2003
Third Edition : 2007
Offices :
India USA
€Bangalore (Phone : 080-26 61 15 61)€Boston €Chennai (Phone : 044-24 34 47 26)11, Leavitt Street, Hingham, €Cochin (Phone : 0484-239 70 04)MA 02043, USA €Guwahati (Phones : 0361-254 36 69, 251 38 81)Phone : 781-740-4487 €Hyderabad (Phone : 040-24 75 02 47) €Jalandhar (Phone : 0181-222 12 72) €Kolkata (Phones : 033-22 27 37 73, 22 27 52 47) €Lucknow (Phone : 0522-220 95 78) €Mumbai (Phones : 022-24 91 54 15, 24 92 78 69) €Ranchi (Phone : 0651-230 77 64)EET-0556-350-ENGG THERMODYNAMICSC - 12751/06/07
Typeset at : Goswami Printers, DelhiPrinted at : Ajit Printers, Delhi DHARMM-therm\TITLE.PM5 v
Preface to The Third Edition
I am pleased to present the third edition of this book. The warm reception which the previous editions and reprints of this book have enjoyed all over India and abroad has been a matter of great satisfaction to me. The entire book has been thoroughly revised ; a large number of solved e xamples (questions having been selected from various universities and competitive examinati ons) and ample additional text have been added. Any suggestions for the improvement of the book will be thankfully ackno wledged and incorporated in the next edition. - AuthorPreface to The First Edition
Several books are available in the market on the subject of "Engineer ing Thermo- dynamics" but either they are too bulky or are miserly written and as such do not cover the syllabii of various Indian Universities effectively. Hence a book is nee ded which should assimilate subject matter that should primarily satisfy the requirements of the students from syllabus/examination point of view ; these requirements are completely m et by this book.The book entails the following features :
- The presentation of the subject matter is very systematic and language o f the text is quite lucid and simple to understand. - A number of figures have been added in each chapter to make the subject matter self speaking to a great extent. - A large number of properly graded examples have been added in various ch apters to enable the students to attempt different types of questions in the ex amination without any difficulty. - Highlights, objective type questions, theoretical questions, and unsolve d examples have been added at the end of each chapter to make the book a complete u nit in all respects. The author's thanks are due to his wife Ramesh Rajput for rendering a ll assistance during preparation and proof reading of the book. The author is thankful to Mr. R.K. Syal for drawing beautiful and well proportioned figures for the book. The author is grateful to M/s Laxmi Publications for taking lot of pains in bringing out the book in time and pricing it moderately inspite of heavy cost of the printing. Constructive criticism is most welcome from the readers. - AuthorContents
ChapterPages
Introduction to S.I. Units and Conversion Factors(xvi) - (xx)Nomenclature(xxi) - (xxii)
1. INTRODUCTION - OUTLINE OF SOME DESCRIPTIVE SYSTEMS ... 1 - 13
1.1.Steam Power Plant...1
1.1.1. Layout...1
1.1.2.Components of a modern steam power plant...2
1.2.Nuclear Power Plant...3
1.3.Internal Combustion Engines...4
1.3.1.Heat engines...4
1.3.2.Development of I.C. engines...4
1.3.3.Different parts of I.C. engines...4
1.3.4.Spark ignition (S.I.) engines...5
1.3.5.Compression ignition (C.I.) engines...7
1.4.Gas Turbines...7
1.4.1.General aspects...7
1.4.2.Classification of gas turbines...8
1.4.3.Merits and demerits of gas turbines...8
1.4.4. A simple gas turbine plant...9
1.4.5.Energy cycle for a simple-cycle gas turbine... 10
1.5.Refrigeration Systems... 10
Highlights... 12
Theoretical Questions... 13
2. BASIC CONCEPTS OF THERMODYNAMICS...14 - 62
2.1.Introduction to Kinetic Theory of Gases... 14
2.2.Definition of Thermodynamics... 18
2.3.Thermodynamic Systems... 18
2.3.1.System, boundary and surroundings... 18
2.3.2.Closed system... 18
2.3.3.Open system... 19
2.3.4.Isolated system... 19
2.3.5.Adiabatic system... 19
2.3.6.Homogeneous system... 19
2.3.7.Heterogeneous system... 19
2.4.Macroscopic and Microscopic Points of View... 19
2.5.Pure Substance... 20
2.6.Thermodynamic Equilibrium... 20
2.7. Properties of Systems... 21
2.8. State... 21
DHARMM-therm\TITLE.PM5 v i i
ChapterPages
( vii )2.9. Process ... 21
2.10. Cycle... 22
2.11.Point Function... 22
2.12.Path Function... 22
2.13. Temperature... 23
2.14.Zeroth Law of Thermodynamics... 23
2.15.The Thermometer and Thermometric Property... 24
2.15.1. Introduction... 24
2.15.2.Measurement of temperature... 24
2.15.3.The international practical temperature scale...31
2.15.4.Ideal gas... 33
2.16. Pressure... 33
2.16.1.Definition of pressure... 33
2.16.2.Unit for pressure... 34
2.16.3.Types of pressure measurement devices...34
2.16.4.Mechanical type instruments... 34
2.17.Specific Volume... 45
2.18.Reversible and Irreversible Processes... 46
2.19.Energy, Work and Heat... 46
2.19.1. Energy... 46
2.19.2.Work and heat... 46
2.20.Reversible Work... 48
Highlights... 58
Objective Type Questions... 59
Theoretical Questions... 61
Unsolved Examples... 61
3. PROPERTIES OF PURE
SUBSTANCES... 63 - 100
3.1.Definition of the Pure Substance... 63
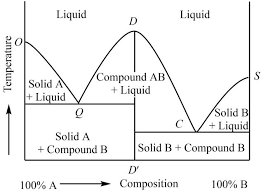
3.2. Phase Change of a Pure Substance... 64
3.3.p-T (Pressure-temperature) Diagram for a Pure Substance... 66
3.4.p-V-T (Pressure-Volume-Temperature) Surface... 67
3.5.Phase Change Terminology and Definitions... 67
3.6.Property Diagrams in Common Use... 68
3.7.Formation of Steam... 68
3.8.Important Terms Relating to Steam Formation... 70
3.9.Thermodynamic Properties of Steam and Steam Tables... 72
3.10.External Work Done During Evaporation... 73
3.11.Internal Latent Heat... 73
3.12.Internal Energy of Steam... 73
3.13.Entropy of Water... 73
3.14.Entropy of Evaporation... 73
3.15.Entropy of Wet Steam... 74
3.16.Entropy of Superheated Steam... 74
3.17.Enthalpy-Entropy (h-s) Chart or Mollier Diagram... 75
DHARMM-therm\TITLE.PM5viii
ChapterPages
( viii )3.18.Determination of Dryness Fraction of Steam... 89
3.18.1.Tank or bucket calorimeter... 89
3.18.2.Throttling calorimeter... 92
3.18.3.Separating and throttling calorimeter... 93
Highlights... 96
Objective Type Questions... 97
Theoretical Questions... 99
Unsolved Examples... 99
4. FIRST LAW OF THERMODYNAMICS...101 - 226
quotesdbs_dbs14.pdfusesText_20[PDF] thermodynamics open system problems and solutions
[PDF] thermodynamics solution
[PDF] thermodynamics steam table problems pdf
[PDF] thermodynamics: closed system problems and solutions
[PDF] thermodynamique chimique smc s4 pdf
[PDF] thermodynamique cours pdf
[PDF] thermodynamique cours pdf mpsi
[PDF] thermodynamique cours pdf s1 fst
[PDF] thermodynamique cours pdf s2
[PDF] these expenses change depending on the good and services you consume
[PDF] these findings suggest synonym
[PDF] thesis about skin care products
[PDF] thesis and assignment writing pdf
[PDF] thesis for annotated bibliography
