 ME 416
ME 416
Italicized versions read from steam tables Bold numbers are calculated. Page 4. ME 201 Thermodynamics. 4. At state 1 we know the pressure and the temperature.
 SOLVED PROBLEMS ON STEAM PROPERTIES
SOLVED PROBLEMS ON STEAM PROPERTIES
From saturated water table of temperature scale at 220°C hf = 943.7 kJ/kg. Page 11. DEPARTMENT OF MECHANICAL ENGINEERING. ME3391 ENGINEERING THERMODYNAMICS.
 Thermodynamics Tables and Charts
Thermodynamics Tables and Charts
From NBS/NRC Steam Tables/1 by Lester Haar John S. Gallagher
 Untitled
Untitled
Review Problems. To find the value for h₂ enter the steam tables. At 5 the pressure is known (P = 0.588 MPa) and the state of the steam is given as saturated.
 SOLUTIONS THERMODYNAMICS PRACTICE PROBLEMS FOR
SOLUTIONS THERMODYNAMICS PRACTICE PROBLEMS FOR
from Mollier diagram or steam table h. 895 from intersection of constant entropy ideal. Btu real lbm turbine ideal stm exh. Btu lbm. Btu lbm w w h h η.
 steam tables - properties of saturated and superheated steam
steam tables - properties of saturated and superheated steam
Table 1. Saturated Steam: Temperature Table-Continued. 10. 10. Abs Press. Specific Volume. Enthalpy. Entropy. Temp. Lb per. Fahr. Sq In. Sat. Liquid. Sat. Evap.
 introduction to chemical engineering thermodynamics
introduction to chemical engineering thermodynamics
%20Hendrick%20Van%20Ness
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
Oct 31 2023 The objective of the course is to survey practical and theoretical problems in classical thermodynamics. ... Table 2.2
 Solutions Manual for Thermodynamics and Chemistry
Solutions Manual for Thermodynamics and Chemistry
Jun 9 2020 Each problem printed in the text is reproduced in this manual
 AREN 2020
AREN 2020
For water using the thermodynamic property tables in the appendices (A-4
 Thermodynamics Basics: Enthalpy Entropy
Thermodynamics Basics: Enthalpy Entropy
https://www.cedengineering.com/userfiles/Thermodynamics%20Basics
 SOLUTIONS THERMODYNAMICS PRACTICE PROBLEMS FOR
SOLUTIONS THERMODYNAMICS PRACTICE PROBLEMS FOR
THERMODYNAMICS PRACTICE PROBLEMS FOR NON-TECHNICAL MAJORS Use the excerpt from the steam tables in Appendix A (Figure A-2) to find h ?
 THERMODYNAMICS HEAT TRANSFER
THERMODYNAMICS HEAT TRANSFER
https://sites.ntc.doe.gov/partners/tr/Training%20Textbooks/06-Thermodynamics
 ME 416
ME 416
Thermodynamics. Practice Problems for Property Evaluation Italicized versions read from steam tables ... ME 201 Thermodynamics.
 Chemical Engineering Thermodynamics II
Chemical Engineering Thermodynamics II
C and 1 atm to water vapor (steam) at the same temperature and pressure. Table 3.2-1 Thermodynamic properties of saturated water.
 Untitled
Untitled
Thermodynamics and. Q = 0.5(2804.8-1
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
Feb 14 2010 gines; image from W. J. M. Rankine
 Solution method for Practice Problems in Problem Sets E F
Solution method for Practice Problems in Problem Sets E F
http://web.mit.edu/10.213/www/solutions/ps-efgp.pdf
 Thermodynamics
Thermodynamics
SOLUTION MANUAL. SI UNIT PROBLEMS. CHAPTER 2. FUNDAMENTALS of. Thermodynamics. Sixth Edition. SONNTAG • BORGNAKKE • VAN WYLEN. CONTENT. SUBSECTION. PROB NO.
 ME 24-221 THERMODYNAMICS I Solutions to extra problems in
ME 24-221 THERMODYNAMICS I Solutions to extra problems in
Nov 29 2000 11.102 Repeat Problem 11.101
 Using the Thermodynamics Steam Table: Crucial Tool for Engineers
Using the Thermodynamics Steam Table: Crucial Tool for Engineers
Saturated Steam: TEMPERATURE Table STEAM TABLES ( from M D Koretsky "Engineering and Chemical Thermodynamics" John Wiley & Sons 2004)
 THERMODYNAMICS PRACTICE PROBLEMS 1 - UTRGV
THERMODYNAMICS PRACTICE PROBLEMS 1 - UTRGV
THERMODYNAMICS PRACTICE PROBLEMS A Carnot refrigerator has a coefficient of performance of 10 If the refrigerator’s interior is to be kept at –45°C the temperature of the refrigerator’s high temperature reservoir is most nearly 250K 270K 300K 350K Solution For a refrigerator COP low = T high?Tlow Solve for the hot side temperature
 Properties of Water and Steam (Thermodynamic Properties of
Properties of Water and Steam (Thermodynamic Properties of
Properties of Water and Steam (Thermodynamic Properties of Ordinary Water Substance) Based onthe NIST Steam Tables For ME209 Thermodynamics at IITBombay Indian Institute of Technology Bombay 22 July 2016 y
 Thermodynamics Tables and Charts - engineeringwayneedu
Thermodynamics Tables and Charts - engineeringwayneedu
Thermodynamics Tables and Charts Table A–1Molar mass gas constant and critical-point properties Table A–2Ideal-gas specific heats of various common gases Table A–3Properties of common liquids solids and foods Table A–4Saturated water—Temperature table
 Searches related to thermodynamics steam table problems pdf PDF
Searches related to thermodynamics steam table problems pdf PDF
Table 1 Saturated Specific Volume Steam: Temperature Table— Continued Sat Vapor 1 4704 1 4667 1 4629 1 4592 1 4555 1 4518 1 4481 1 4444 1 4407 1 4370 1 4333
What are thermodynamics steam tables?
The thermodynamics steam tables contain the following tables: Saturated water and steam temperature tables: In these tables for every temperature the absolute pressure, specific volume for saturated water and saturated steam, specific enthalpy for saturated water and saturated steam and specific entropy for saturated and saturated steam are given.
How do I configure steam/water thermodynamic properties?
Steam/Water Thermodynamic Properties Calculations Program User Manual 3 CONFIGURATION The Steam/Water Properties program requires you to select a phase option on the Steam/Water Properties Setup screen. The flowing pressure and temperature for calculating the steam or water properties are those configured for the FloBoss 103.
Why are thermodynamic properties presented in the form of tables?
For most substances, thermodynamic properties are presented in the form of tables because they are too complex to be expressed by simple equations. In thermo-systems, many working fluids can be used. Water is one of the most common working fluids involved. It is the only liquid presented in this section.
What are the units used in the steam/water thermodynamic properties calculator?
The program sets the density at base conditions to the density at flowing conditions, since setting base conditions for steam and water flow rate measurements is not a common practice. Units are Lb/CF or Kg/M 3 47,0,25 Steam/Water Thermodynamic Properties Calculations Program User Manual Revised Mar-08 18
SOLUTIONS
THERMODYNAMICS PRACTICE PROBLEMS FOR NON-TECHNICAL MAJORSThermodynamic Properties
1. If an object has a weight of 10 lbf on the moon, what would the same object
weigh on Jupiter?JupiterMo
onc 22ftftlbm-ft g=75g=5.4g=32 secseclbf-sec 2 c moon cmoon
Jupiter
Jupiter
c Wg mg10×32W=m = = = 59.26 lbm
gg5.4 mg59.26×75
W = = = 139 lbf
g322. An object that weighs 50 lbf on earth is moved to Saturn where its new weight is
105 lbf. What is the acceleration due to gravity on Saturn?
Earthc22
ftlbm-ft g=32g=32 seclbf-s ec c 2 c 5050Wg mg10532
W=67.2
gm50s lbfonearthlbm ft g ec3. Define, using equations, specific volume ( and density()Ȟ)ȡ. What is the
mathematical relationship between these two terms? 11 Vm or mVTemperature and Pressure Measurements
4. (a) Define temperature.
(b) What is the absolute temperature scale corresponding to Fahrenheit? (c) Convert 100° F to that absolute scale. (a) Temperature: a measure of molecular activity of a substance. (b) Rankine (c) °R = °F + 460 100° F converts to 560° RPage 1 of 9
5. Define pressure.
Pressure: a measure of force exerted per unit area on the boundaries of a system.6. If P
A =P B , in which direction will the piston move? Explain, using equations. A B F P= A F P= APiston will move to the left.
BABA AAFFF7. Given: P
1 = 4 psig, P ATM = 15 psia, and P 2 = 10 psigFind P
A and P B. ATM gagesystemreference1ATMBB
2ABAP = P - P
P = P - PP = 15 psia - 4 psia = 11 psia
P = P - P P = 10 psig + 11 psia = 21 psia
Page 2 of 9
8. Given: P
ATM = 15 psia, P 2 =6 psiv, and P 3 = 7 psigFind P
A and P B. ATM gagesystemreference3AATMA
2ABBP = P - P
P = P - PP = 15 psia + 7 psia = 22 psia
P = P - P P = 22 psia - (- 6) psi = 28 psia
9. Given the conversion factor 1 inch H
2O = 0.0361 psid and that the manometer
below employs water, find the difference in pressure between compartments A and B. 6 ft B A6ft12in0.0361psid
ǻP = = 2.6 psid
1ft1in
Energy, Work, and Heat
10. Define energy.
Energy: the capacity of a system to perform work or produce heat.Page 3 of 9
11. Define, using equations, the total kinetic energy, total potential energy, and
enthalpy. 2 2 c c mgz PE g mv KE g huPν12. Given the following information about a system, calculate specific enthalpy (in
Btu/lbm).
3 ftBtuP=100psia=1.6u=600Note:778ft-lbf=1Btu
lbmlbm 3222
144
600(100)(1.6)()()629.6
778νhuP
BtulbfftinBtuBtu
h lbminlbmftftlbflbm13. Given the following information about a system, calculate specific internal energy
(in Btu/lbm). 3 ftBtuP=200psia=2.8h=1000Note:778ft-lbf=1Btu
lbmlbm 3222
144
1000(200)(2.8)()()896.3
778ννhuPuhP
BtulbfftinBtuBtu
u lbminlbmftftlbflbm14. A 5 lbm system was taken from 50° F to 150° F. How much energy in the form of
heat was added to the system to produce this temperature increase? p Btu c=1.6 lbm-F photcoldQmc(TT)
BtuQ5lbm1.6(15050)F800Btu
lbmF15. A 10 lbm metal ball has a temperature of 200° F when it is placed in a 50 lbm
bath of water at room temperature (72° F). Heat transfer occurs between the two substances until equilibrium is reached. Find this equilibrium temperature.WaterMetal
ppBtuBtu
c=1.0c=4.3 lbm-Flbm-FPage 4 of 9
104.3200501.072
501.0104.3
BallWater
initialinitial OutIn ballballballeqwaterwatereqwater ballwater eq ballwater eq eq QQ mcTTmcTT mcTmcT T mcmc lbmBtuFlbmBtuF lbmFlbmF T lbmBtulbmBtu lbmFlbmFT131.2F
16. During a phase change, the specific entropy of a 20 lbm system increases from
0.31 Btu lbmR to 1.61 Btu lbmR while the temperature of the substance is a constant212°F.
Find the heat transfer into this system.
Hint: Must convert temperature to Rankine.
()()212460R1.610.31Btu 20lbmQmTs17,472Btu
lbmR17. A nuclear power plant is found to generate 80 MW of power. A typical Honda
civic is capable of producing 150 HP. How many Honda Civic's would be required to generate the equivalent power of this nuclear power plant? Use the energy and power equivalences found in the DOE Fundamentals Handbook (see Pages 23 and 24 of the "Energy, Work, and Heat" module).80MW1000KW3,413BTU1HPhr1HondaCivic
715.23716HondaCivics
1MW1KWhr2,545BTU150HP
Thermodynamic Systems and Processes
18. Define isolated system, closed system, and open system.
Isolated system - A system that is not influenced in any way by its surroundings (mass and energy do not cross the system boundary). Closed System - A system which has no transfer of mass with its surroundings, but that may have a transfer of energy. Open System - A system that may have a transfer of both mass and energy with its surroundings19. Can a system be in steady state yet have the fluid passing through it undergoing
a phase change? Reconcile your answer with the definition of steady state.Page 5 of 9
Yes. Steady state occurs in a system when the fluid properties at a given point remain constant with respect to time. A fluid undergoing a phase change will have properties that change from point to point. However, to determine if the system is in steady state, we must concentrate on a single point over time.Change of Phase
20. Describe the difference between an intensive and an extensive property. Give 2
examples of each type of property. Intensive properties are independent of the amount of mass present. Extensive properties are a function of the amount of mass present. Examples of intensive properties are pressure, temperature, and density. Examples of extensive properties are volume, weight, and energy.21. A system contains 250 lbm of saturated liquid and 10 lbm of saturated vapor.
What is the quality of the system?
100.0383.8%
+ 250 + 10 vapor vaporliquid m lbm Xo mmlbmlbm rProperty Diagrams and Steam Tables
22. Steam enters a turboexpander as a saturated vapor at 500 psia and is expanded
at constant entropy to 5 psia. Using the Mollier diagram in Appendix A (Figure A-1), find the Δh for this process.
From the Mollier diagram:
Btu1205895310
lbm23. Use the excerpt from the steam tables in Appendix A (Figure A-2) to find h, Ȟ,
and s for water:Saturated liquid, P = 350 psia
3409.80.019120.6059===
BtuftBtu
hs lbmlbmlbmRSaturated vapor, P = 400 psia
31204.61.160951.4847===
BtuftBtu
hs lbmlbmlbmRSaturated liquid, T = 468 F
3450.70.019760.6502===
BtuftBtu
hs lbmlbmlbmRSuperheated steam, P = 400 psia and T=700 F
31363.41.64991.6406===
BtuftBtu
hs lbmlbmlbmRPage 6 of 9
24. Use the steam tables and the concept of quality to find h and ν for water at a
pressure of 260 psia if entropy is known to be 0.725 Btu lbmR 30.7250.5722
16%0.9508
379.90.16821.6511.4
0.018700.161.755480.29958ννν
WVf WVffg fg WVffg WVffg ss ssXsX s Btu hhXh lbm ft X lbm25. Calculate specific internal energy for a 200 psia system of saturated liquid.
Hint: Review the definition of enthalpy.
3222
144
355.5(200)(0.01839)()()354.82
778ννhuPuhP
BtulbfftinBtuBtu
u lbminlbmftftlbflbmFirst Law of Thermodynamics
26. State the First Law of Thermodynamics.
Energy can neither be created nor destroyed, only altered in form.27. The following schematic of a simple Rankine cycle consists of steam leaving a
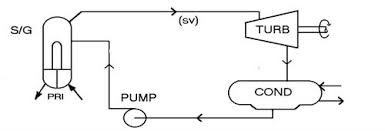
boiler at T=550 F and P=400 psia and passes through a turboexpander where it does work and exhausts with an enthalpy of 932 Btu/lbm. The exhaust is then condensed to an enthalpy of 85 Btu/lbm before being pumped back into the boiler. h=932 Btu/lbmT=550 F
P=400 psia
h=?? Btu/lbm T116F h=85 Btu/lbm 67turbboiler systemCond
BtuBtu
Given W=4.15×10 and Q=1.43×10,find the mass flow rate of the lbmlbm system(m), the total heat transfer out at the condenser(Q),and the enthalpy of the fluid after leavin g the pump and before entering the boiler.Page 7 of 9
6 4 474.1510
1.210 (1277.5932) ()1.210(93285)1.0210quotesdbs_dbs14.pdfusesText_20[PDF] thermodynamique chimique smc s4 pdf
[PDF] thermodynamique cours pdf
[PDF] thermodynamique cours pdf mpsi
[PDF] thermodynamique cours pdf s1 fst
[PDF] thermodynamique cours pdf s2
[PDF] these expenses change depending on the good and services you consume
[PDF] these findings suggest synonym
[PDF] thesis about skin care products
[PDF] thesis and assignment writing pdf
[PDF] thesis for annotated bibliography
[PDF] thesis formula
[PDF] thesis of 13th documentary
[PDF] thesis on creative writing pdf
[PDF] thesis on unemployment in the philippines
