 Experiment 25: Calorimetry
Experiment 25: Calorimetry
CHEM 111 Morning Lab. 27 October 2014. Experiment 25: Calorimetry. Conclusion: The unknown metal #14 has a specific heat of 0.36 J/g °C; the heat of
 Coffee Cup Calorimetry
Coffee Cup Calorimetry
Read through the sample procedures data
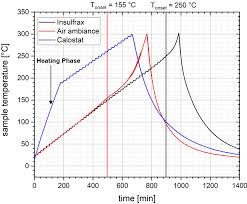 Experience Report on Adiabatic Reaction Calorimetry in Safety
Experience Report on Adiabatic Reaction Calorimetry in Safety
In conclusion the evaluation method has no significant influence on the In summary it has to pointed out that an interpretation of an adiabatic experiment ...
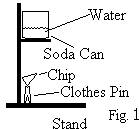
 Soda Can Calorimeter
Soda Can Calorimeter
In the 1770s Joseph Black (1728–1799) was one of the first scientists to conduct calorimetry experiments with different materials. He discovered that not
 Experiment 8 Calorimetry
Experiment 8 Calorimetry
This experiment will require most of the time allocated to the lab period. If you Report: The laboratory report should include Tables 1-4 (add titles to ...
 Calorimetry Lab Report On Planters Cashews Chemistry Period 3
Calorimetry Lab Report On Planters Cashews Chemistry Period 3
2 дек. 2015 г. Conclusion. The hypothesis created at the beginning the lab was incorrect. Throughout the attempts of finding the amount of calories in a ...
 AP CHEMISTRY SYLLABUS PHS 2017-2018
AP CHEMISTRY SYLLABUS PHS 2017-2018
Notebook Laboratory Reports- Always include the following in each report: Title The title should be descriptive. Experiment 5 is not a descriptive title. Date
 Example Calorimetry Lab Report #2 – Good or In Need of Lots of
Example Calorimetry Lab Report #2 – Good or In Need of Lots of
26 окт. 2010 г. In this experiment the differences in caloric content of foods were investigated through the ignition of food items and the measurement of the ...
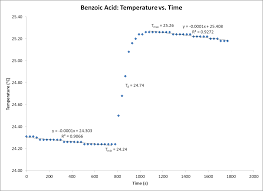 Physical Chemistry Lab Report Rubric –Veldman Fall 2012
Physical Chemistry Lab Report Rubric –Veldman Fall 2012
We can conclude that the use of bomb calorimetry to determine the heat of combustion of sucrose was successful despite moderate experimental error. 3. Page 5
 Experiment 15: Specific Heat of a Metal
Experiment 15: Specific Heat of a Metal
Sep 5 2013 Conclusions: The specific heat of two metals was determined experimentally. The technique utilized was calorimetry. A sample of each metal was ...
 Writing a discussion
Writing a discussion
The discussion is the most important part of the laboratory report because it is here that you discussion for an introductory chemistry experiment.
 Example Calorimetry Lab Report #2 – Good or In Need of Lots of
Example Calorimetry Lab Report #2 – Good or In Need of Lots of
Oct 26 2010 In this experiment
 Experiment 25: Calorimetry
Experiment 25: Calorimetry
CHEM 111 Morning Lab. 27 October 2014. Experiment 25: Calorimetry. Conclusion: The unknown metal #14 has a specific heat of 0.36 J/g °C;
 Experiment 6 ? Calorimetry
Experiment 6 ? Calorimetry
Pre?lab questions Calorimetry is the science of measuring the quantities of heat ... customary to report its value on a per?mole basis. Thus we are.
 Finding the Specific Heat of a Substance
Finding the Specific Heat of a Substance
To measure specific heat in the laboratory a calorimeter of some kind must be In this experiment
 Coffee Cup Calorimetry
Coffee Cup Calorimetry
discussion of Pre-Lab Exercises regarding the difference in temperature is measured in calorimetry experiments. The ... and report its units.
 Bomb Calorimetry and Heat of Combustion
Bomb Calorimetry and Heat of Combustion
Nov 7 2014 In this experiment we used a Parr bomb calorimeter to accurately ... Raw data for this experiment is included in the Results section
 EXPERIMENT 12N CALORIMETRY
EXPERIMENT 12N CALORIMETRY
Aug 7 2018 Read the entire experiment and instructions
[PDF] calweasel past exams
[PDF] cambridge curriculum for kindergarten
[PDF] cambridge curriculum guide
[PDF] cambridge curriculum online
[PDF] cambridge curriculum primary
[PDF] cambridge curriculum review
[PDF] cambridge curriculum schools in south africa
[PDF] cambridge curriculum south africa
[PDF] cambridge curriculum vs ib
[PDF] cambridge delta course locations
[PDF] cambridge english
[PDF] cambridge english course book
[PDF] cambridge english course book 9
[PDF] cambridge english course book pdf
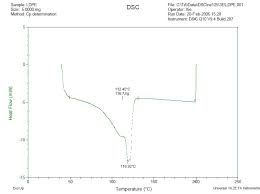 NE 125 - Experiment 3
NE 125 - Experiment 3