 Coly-Mycin M Parenteral (Colistimethate for Injection USP)
Coly-Mycin M Parenteral (Colistimethate for Injection USP)
Each vial contains colistimethate sodium or pentasodium colistinmethanesulfonate (150 mg colistin base activity). Colistimethate sodium is a polypeptide
 Coly-Mycin M Parenteral
Coly-Mycin M Parenteral
Each vial contains colistimethate sodium or pentasodium colistinmethanesulfonate (150 mg colistin base activity). Colistimethate sodium is a polypeptide
 Colistimethate for Injection USP 150 mg (base) / vial
Colistimethate for Injection USP 150 mg (base) / vial
26-Feb-1999 Colistimethate sodium has the following structural for- mula: mcg/mL colistin base equivalent. 1000. CLINICAL PHARMACOLOGY. Microbiology ...
 Colobreathe INN: colistimethate sodium
Colobreathe INN: colistimethate sodium
10-Oct-2011 colistimethate sodium (rinn). Procedure No.: EMEA/H/C/001225. Note. Assessment report as adopted by the CHMP with all information of a ...
 Colobreathe INN-colistimethate sodium
Colobreathe INN-colistimethate sodium
13-Feb-2012 Each capsule contains 1662
 PATIENT INFORMATION LEAFLET - 1 million international units/vial
PATIENT INFORMATION LEAFLET - 1 million international units/vial
The name of your medicine is Colistimethate sodium 1 Million I.U. Powder for Solution for. Injection. It is referred to as Colistimethate in this leaflet. Read
 Colistimethate Sodium (Intravenous) Monograph
Colistimethate Sodium (Intravenous) Monograph
The following table may be a useful guide. International units of. Colistimethate sodium. Colistin base. 1 million. 33.33 mg. • Calculate dosage for obese
 Colistimethate sodium for injection BP - KOOLISTINTM
Colistimethate sodium for injection BP - KOOLISTINTM
Because colistimethate sodium is largely excreted in the urine dose reduction is required in renal impairment to prevent accumulation. After intravenous
 High Dose Colistimethate Sodium (Colistin) in Adults – Consensus
High Dose Colistimethate Sodium (Colistin) in Adults – Consensus
This guidance does not cover use of CMS for respiratory infections in cystic fibrosis patients. Terminology: Colistimethate sodium (CMS) is a non-active pro-
 A Novel Validated Injectable Colistimethate Sodium Analysis
A Novel Validated Injectable Colistimethate Sodium Analysis
11-Mar-2021 Colistin can be administered as colistin sulfate (CS) either orally or topically and as col- istimethate sodium (CMS) parenterally or by ...
 [PDF] colistimethate sodium
[PDF] colistimethate sodium
(colistimethate sodium) Poudre pour solution (équivalent à 150 mg de colistine base) Antibiotique antibactériens SteriMax Inc 1-2735 Boul Matheson E
 [PDF] Colobreathe colistimethate sodium
[PDF] Colobreathe colistimethate sodium
Colistimethate sodium and colistin have been investigated in vitro to determine the effects on the expression of cytochrome P450 (CYP) enzymes on treating
 [PDF] Colobreathe
[PDF] Colobreathe
13 fév 2012 · Colobreathe contient du colistiméthate de sodium un antibiotique de la famille des polymyxines Colobreathe est utilisé pour contrôler les
 [PDF] Coly-Mycin M Parenteral (Colistimethate for Injection USP)
[PDF] Coly-Mycin M Parenteral (Colistimethate for Injection USP)
Colistimethate sodium is a polypeptide antibiotic with an approximate molecular weight of 1750 The empirical formula is C58H105N16Na5O28S5 and the
 [PDF] Colistimethate Sodium (Intravenous) Monograph - Paediatric
[PDF] Colistimethate Sodium (Intravenous) Monograph - Paediatric
Available at PCH: Intravenous injection: • 150mg colistin base (equivalent to 4 5 million units of colistimethate sodium) powder for injection Inhalation:
 [PDF] COLIMYCINE - CT-4947
[PDF] COLIMYCINE - CT-4947
colistiméthate sodique pour COLIMYCINE 1 000 000 UI poudre et solvant pour solution Giamarellou H Interactions of colistin and rifampin on multidrug-
 [PDF] Colistimethate sodium for injection BP - Biocon
[PDF] Colistimethate sodium for injection BP - Biocon
Colistimethate sodium is a cyclic polypeptide antibiotic derived from Bacillus polymyxa var colistinus and belongs to the polymyxin group CLINICAL
 [PDF] ADMINISTRATION DE COLISTIN® EN AEROSOL ET EN IV
[PDF] ADMINISTRATION DE COLISTIN® EN AEROSOL ET EN IV
1 jui 2012 · La Colistin est indiquée pour le traitement des infections des voies respiratoires germes Gram-négatifs sensible (ex Pseudomonas aeruginosa
 [PDF] Colistimethate sodium and acute kidney injury - Nefrología
[PDF] Colistimethate sodium and acute kidney injury - Nefrología
28 mai 2020 · Background: Colistimethate sodium (CMS) treatment has increased over the last years being acute kidney injury (AKI) its main drug-related
 [PDF] High Dose Colistimethate Sodium (Colistin) in Adults
[PDF] High Dose Colistimethate Sodium (Colistin) in Adults
Colistimethate sodium (CMS) exhibits concentration-dependent bactericidal nbt nhs uk/wp-content/uploads/Antibiotic-Assay-Guideline-Ranges-20151 pdf
 O N
O N CR L-Dbu L-Thr L-Dbu L-Dbu
N N N N N
CH 2 CH 2 CH 2 CH 2 CH 2O S O O S O O S O O S O O S O
ON a ON a ON a ON a ON aL-ThrL-Dbu D-Leu L-Leu L-Dbu L-Dbu
NDA 50-108/S-026
Page 3
Coly-Mycin
M Parenteral
(Colistimethate for Injection, USP) To reduce the development of drug-resistant bacteria and maintain the effectiveness of Coly-Mycin Mand other antibacterial drugs, Coly-Mycin M should be used only to treat or prevent infections that are
proven or strongly suspected to be caused by bacteria.FOR INTRAMUSCULAR AND INTRAVENOUS USE
DESCRIPTION
Coly-Mycin® M Parenteral (Colistimethate for Injection, USP) is a sterile parenteral antibiotic product
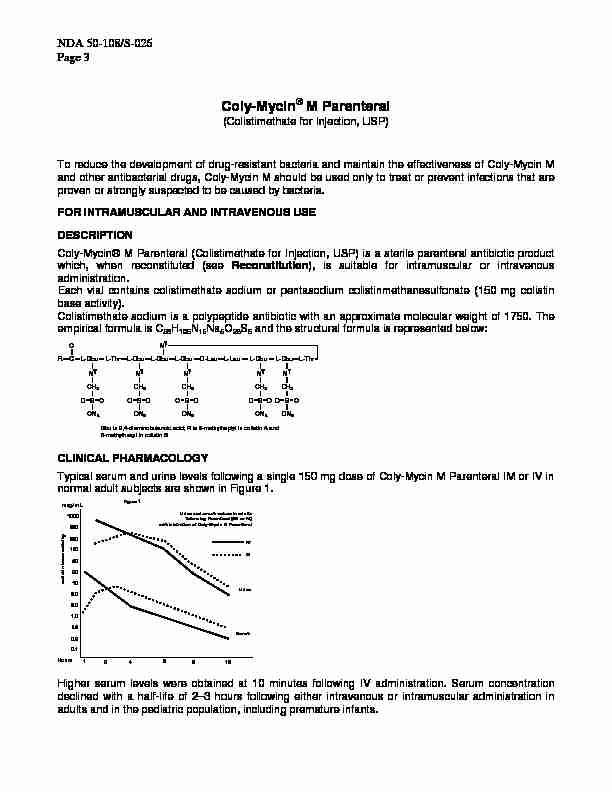
which, when reconstituted (see Reconstitution), is suitable for intramuscular or intravenous administration. Each vial contains colistimethate sodium or pentasodium colistinmethanesulfonate (150 mg colistin base activity). Colistimethate sodium is a polypeptide antibiotic with an approximate molecular weight of 1750. The empirical formula is C58 H 105N 16 Na 5 O 28
S 5 and the structural formula is represented below: Dbu is 2,4-diaminobutanoic acid; R is 5-methylheptyl in colistin A and
5-methylhexyl in colistin B
CLINICAL PHARMACOLOGY
Typical serum and urine levels following a single 150 mg dose of Coly-Mycin M Parenteral IM or IV in normal adult subjects are shown in Figure 1.Figure 1
mcg/mL Urine and serum values in adults following Parenteral (IM or IV) administration of Coly-Mycin M Parenteral IV IM Urine Serum 1000500
200
100
50
20 10 5.0 2.0 1.0 0.5 0.2 0.1 colistin base activity
Hours12 4 6 8 12
Higher serum levels were obtained at 10 minutes following IV administration. Serum concentrationdeclined with a half-life of 2-3 hours following either intravenous or intramuscular administration in
adults and in the pediatric population, including premature infants.NDA 50-108/S-026
Page 4
Average urine levels ranged from about 270 mcg/mL at 2 hours to about 15 mcg/mL at 8 hours after intravenous administration and from 200 to about 25 mcg/mL during a similar period following intramuscular administration. Microbiology: Colistimethate sodium is a surface active agent which penetrates into and disrupts the bacterial cell membrane. It has been shown to have bactericidal activity against most strains of the following microorganisms, both in vitro and in clinical infections as described in the INDICATIONSAND USAGE section:
Aerobic gram-negative microorganisms: Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa.Susceptibility Tests:
Colistimethate sodium is no longer listed as an antimicrobial for routine testing and reporting by clinical microbiology laboratories.INDICATIONS AND USAGE
Coly-Mycin M Parenteral is indicated for the treatment of acute or chronic infections due to sensitive
strains of certain gram-negative bacilli. It is particularly indicated when the infection is caused by
sensitive strains of Pseudomonas aeruginosa. This antibiotic is not indicated for infections due toProteus or Neisseria. Coly-Mycin M Parenteral has proven clinically effective in treatment of infections
due to the following gram-negative organisms: Enterobacter aerogenes, Escherichia coli, Klebsiella pneumoniae and Pseudomonas aeruginosa.Coly-Mycin M Parenteral may be used to initiate therapy in serious infections that are suspected to be
due to gram-negative organisms and in the treatment of infections due to susceptible gram-negative pathogenic bacilli. To reduce the development of drug-resistant bacteria and maintain the effectiveness of Coly-Mycin Mand other antibacterial drugs, Coly-Mycin M should be used only to treat or prevent infections that are
proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibilityinformation are available, they should be considered in selecting or modifying antibacterial therapy. In
the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric
selection of therapy.CONTRAINDICATIONS
The use of Coly-Mycin M Parenteral is contraindicated for patients with a history of sensitivity to the
drug or any of its components.WARNINGS
Maximum daily dose should not exceed 5 mg/kg/day (2.3 mg/lb) with normal renal function. Transient neurological disturbances may occur. These include circumoral paresthesia or numbness,tingling or formication of the extremities, generalized pruritus, vertigo, dizziness, and slurring of
speech. For these reasons, patients should be warned not to drive vehicles or use hazardous machinery while on therapy. Reduction of dosage may alleviate symptoms. Therapy need not be discontinued, but such patients should be observed with particular care. Nephrotoxicity can occur and is probably a dose-dependent effect of colistimethate sodium. These manifestations of nephrotoxicity are reversible following discontinuation of the antibiotic.NDA 50-108/S-026
Page 5
Overdosage can result in renal insufficiency, muscle weakness, and apnea (see OVERDOSAGE section). See PRECAUTIONS, Drug Interactions subsection for use concomitantly with other antibiotics and curariform drugs.Respiratory arrest has been reported following intramuscular administration of colistimethate sodium.
Impaired renal function increases the possibility of apnea and neuromuscular blockade following administration of colistimethate sodium. Therefore, it is important to follow recommended dosingquotesdbs_dbs2.pdfusesText_3[PDF] balabala festival paris l été
[PDF] stratégies d'écriture au secondaire
[PDF] play paris l ete
[PDF] stratégies d'écriture au primaire
[PDF] guide denseignement efficace lecture
[PDF] chagall vision de paris analyse
[PDF] delaunay tour eiffel
[PDF] la seine a rencontré paris jacques prévert
[PDF] poésie la seine
[PDF] la seine a rencontré paris prévert date
[PDF] paris at night prévert
[PDF] la seine a rencontré paris date
[PDF] paris at night prévert analyse
[PDF] paris at night poeme
