 Etude quantitative de lévolution dun système Constante déquilibre
Etude quantitative de lévolution dun système Constante déquilibre
En déduire le potentiel rédox des couples en solution. V150). Sn/. Sn(E Les demi-équations électroniques et les formules de Nernst des deux couples ...
 Diagrammes potentiel-pH
Diagrammes potentiel-pH
2 mai 2018 Les espèces prises en compte sont Sn(s). SnO2(s)
 V- EXERCICES :
V- EXERCICES :
Ecrire l'équation bilan de la réaction chimique traduisant le dépôt métallique. Exercice 4 : Ecrire les demi-équations d'oxydoréduction relatives aux
 Oxydant Réducteur E0 (Volt)
Oxydant Réducteur E0 (Volt)
Sn(s). 0.05. HOCN+2H++2 e-. HCN(aq)+ H2O. 0.02. NO3. -+ H2O +2 e-. NO2. -+2OH-. 0.01. 2 H++2 e-. H2(g). 0.00. HOCN+2 H++2 e-. HCN(g)+ H2O. -0.02. Fe3++3 e-.
 Faculté de médecine 2011/
Faculté de médecine 2011/
Quels sont les couples redox mis en jeu ? - écrire les demi équations électroniques. - Ecrire l'équation de la réaction. Quelle est la masse du dépôt de cuivre
 Correction exercice n°2
Correction exercice n°2
2) Demi-équations : SnO2(s) + 4 H+ b) D'après l'équation bilan : n (Sn2+) = n (Pb2+) = 19 × 10-3 mol. Partie 2 : ... on en déduit : n (Sn.
 Les réactions doxydo-réduction
Les réactions doxydo-réduction
3/ Les demi-équations électroniques. ? Chaque couple oxydant-réducteur représente un transfert d'électron(s) réalisable dans les 2 sens.
 Etude cinétique d’une réaction d’oxydoréduction
Etude cinétique d’une réaction d’oxydoréduction
des ions Sn. 2+ à la concentration 10-2 mol.L-1 le temps de demi-réaction T est de 2
 Les piles et loxydo-réduction :
Les piles et loxydo-réduction :
1 - Quel type d'électrodes (ou demi-piles) sont en présence ? Donner l'expression du La demi-pile (B) est du type redox
 Oxydoréduction – corrigé des exercices Table des matières
Oxydoréduction – corrigé des exercices Table des matières
Comment équilibrer les équations des réactions rédox Sn + HNO3 ... Pb(NO3)2 1 mol·L-1 et la deuxième demi-pile avec une solution aqueuse de nitrate.
 Oxidation- Reduction Chemistry - WRUV
Oxidation- Reduction Chemistry - WRUV
1 Write Skeleton Half-Reactions Oxidation SO32-? SO 4 2-Reduction MnO4-? Mn2+ 2 Mass Balance SO3 2-+ H 2O? SO42-+ 2H+ MnO4-+ 8H+ ? Mn2+ + 4H 2O •Add H2O to side needing oxygen •Add H+ to balance hydrogen 6 Example: Continued 3 Charge Balance (use electrons) SO3 2-+ H 2O ? SO42-+ 2H+ + 2e-MnO4-+ 8H+ + 5e-? Mn2+ + 4H 2O 4
 Example Exercise 171 Calculating Oxidation Numbers for Carbon
Example Exercise 171 Calculating Oxidation Numbers for Carbon
A redox reaction occurs when the tin(II) ion reacts with the iodate ion as follows: Indicate each of the following for the preceding redox reaction: (a) substance oxidized (b) substance reduced (c) oxidizing agent (d) reducing agent Answers: (a) Sn2+; (b) IO 3 –; (c) IO 3 –; (d) Sn 2+ Practice Exercise
 Searches related to demi equation redox sn2+/sn PDF
Searches related to demi equation redox sn2+/sn PDF
In our reaction the product formed from Fe2+(aq) is Fe3+(aq) and possible products from the MnO4– ion are Mn2+(aq) Mn3+(aq) MnO2(s) or MnO42– The reaction product and the number of electrons gained by KMnO4 must be known before using the reagent in analytical determinations
How do you balance a redox reaction?
Balance each redox reaction by writing appropriate half reactions and combining them to cancel the electrons. Pb (s) + Pb 4+ (aq) ? Pb 2+ (aq) (Hint: both half reactions will start with the same reactant.) 11.5: Half-Reactions is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts.
How is cell potential calculated if a redox reaction is reversed?
Note that reversing the direction of a redox reaction effectively interchanges the identities of the cathode and anode half-reactions, and so the cell potential is calculated from electrode potentials in the reverse subtraction order than that for the forward reaction.
How redox potentials are used in a galvanic cell?
To use redox potentials to predict whether a reaction is spontaneous. To balance redox reactions using half-reactions. In a galvanic cell, current is produced when electrons flow externally through the circuit from the anode to the cathode because of a difference in potential energy between the two electrodes in the electrochemical cell.
Why does a balanced redox reaction have two electrons on each side?
Because we have two electrons on each side of the equation, they can be canceled. This is the key criterion for a balanced redox reaction: the electrons have to cancel exactly. If we check the charge on both sides of the equation, we see they are the same—2+.
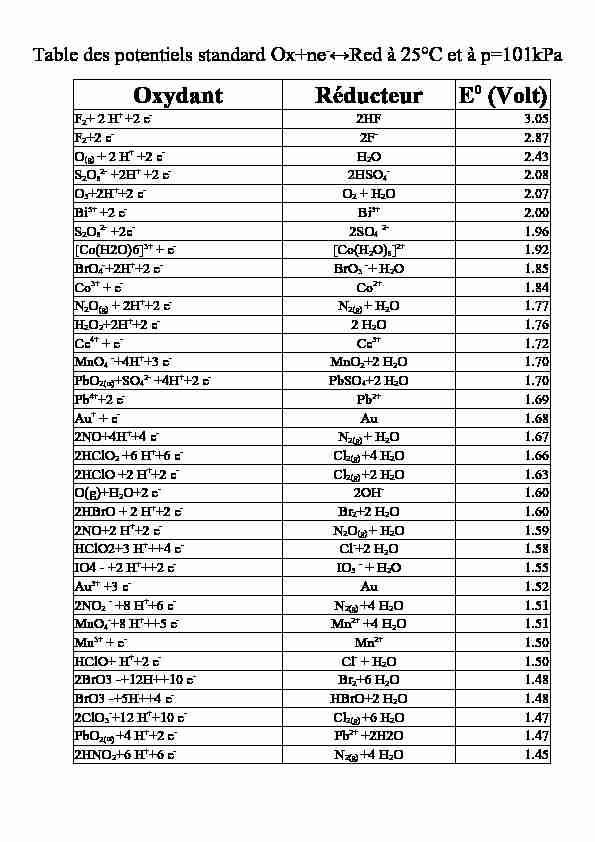
Table des potentiels standard Ox+ne-↔Red à 25°C et à p=101kPaOxydantRéducteurE0 (Volt)F2+ 2 H+ +2 e-2HF3.05F2+2 e-2F-2.87O(g) + 2 H+ +2 e-H2O2.43S2O82- +2H+ +2 e-2HSO4-2.08O3+2H++2 e-O2 + H2O2.07Bi5+ +2 e-Bi3+2.00S2O82- +2e-2SO4 2-1.96[Co(H2O)6]3+ + e-[Co(H2O)6]2+1.92BrO4-+2H++2 e-BrO3 -+ H2O1.85Co3+ + e-Co2+1.84N2O(g) + 2H++2 e-N2(g) + H2O1.77H2O2+2H++2 e-2 H2O1.76Ce4+ + e-Ce3+1.72MnO4 -+4H++3 e-MnO2+2 H2O1.70PbO2(a)+SO42- +4H++2 e-PbSO4+2 H2O1.70Pb4++2 e-Pb2+1.69Au+ + e-Au1.682NO+4H++4 e-N2(g) + H2O1.672HClO2 +6 H++6 e-Cl2(g) +4 H2O1.662HClO +2 H++2 e-Cl2(g) +2 H2O1.63O(g)+H2O+2 e-2OH-1.602HBrO + 2 H++2 e-Br2+2 H2O1.602NO+2 H++2 e-N2O(g) + H2O1.59HClO2+3 H+++4 e-Cl-+2 H2O1.58IO4 - +2 H+++2 e-IO3 - + H2O1.55Au3+ +3 e-Au1.522NO2 - +8 H++6 e-N2(g) +4 H2O1.51MnO4-+8 H+++5 e-Mn2+ +4 H2O1.51Mn3+ + e-Mn2+1.50HClO+ H++2 e-Cl- + H2O1.502BrO3 -+12H++10 e-Br2+6 H2O1.48BrO3 -+5H++4 e-HBrO+2 H2O1.482ClO3-+12 H++10 e-Cl2(g) +6 H2O1.47PbO2(a) +4 H++2 e-Pb2+ +2H2O1.472HNO2+6 H++6 e-N2(g) +4 H2O1.45
Table des potentiels standard Ox+ne-↔Red à 25°C et à p=101kPaOxydantRéducteurE0 (Volt)2NO2-+6 H++4 e-N2O(g) +3 H2O1.40ClO4- +8 H++8 e-Cl-+4 H2O1.39ClO4- +16 H++16 e-Cl2(g) +2 H2O1.39Cl2(g) +2 e-2Cl-1.39HCrO4-+7 H++3 e-Cr3+ +4 H2O1.382NO2(g) +8 H++8 e-N2(g) +4 H2O1.36N2O4(g) +8 H++8 e-N2(g) +4 H2O1.36Cl2(g)+2 e-2Cl-1.36Cr2O72- +14 H++6 e-2Cr3+ +7 H2O1.36HBrO+ H++2 e-Br-+H2O1.34ICl3(s) +2 e-ICl+2Cl-1.2803+H2O+2 e-O2+2OH-1.252NO3-+12 H++10e-N2(g) +6 H2O1.25MnO2+4 H++2 e-Mn2+ +2 H2O1.232NO2(g) +6 H++6 e-N2O(g) +3 H2O1.23O2+4 H++4 e-2 H2O1.23ClO4-+2 H++2e-ClO3-+H2O1.20NO2-+2 H++ e-NO(g) + H2O1.202ICl(aq) +2 e-I2+2Cl-1.202IO3-+12 H++10 e-I2(aq) +6 H2O1.19Pt2+ +2 e-Pt(s)1.19ClO2(g) + H++e-HClO21.19ClO3-+3 H++2 e-HClO2+ H2O1.18ClO3-+2 H++e-ClO2(g) + H2O1.17Pt4+ +4 e-Pt(s)1.152NO3-+10 H++8 e-N2O(g) +5 H2O1.12O2+4 e-2O2-1.12NO2(g)+H++e-HNO21.09Br(aq)+2e-2Br -1.09IO3-+6H++6e-I-+3 H2O1.08N2O4(g)+2H++2e-2HNO21.07Br2(l) +2e-2Br -1.06NO2(g) +2H++2 e-NO(g) + H2O1.05Br3 -+2e-3Br -1.05N2O4(g) +4H++4 e-2NO(g) + H2O1.04
Table des potentiels standard Ox+ne-↔Red à 25°C et à p=101kPaOxydantRéducteurE0 (Volt)HNO2+H++ e-NO(g) + H2O1.00VO2 ++2H++ e-VO2++ H2O1.00Pd2++2e-Pd(s)0.99HIO(aq) +H++2 e-I-+ H2O0.98CNO-+H2O+2 e-CN-+2OH-0.97NO3- (HNO3 30%) +4H++3 e-NO(g)+2 H2O0.94HgO(r)+2H++2 e-Hg(l)+ H2O0.932Hg2+ (aq) +2 e-Hg22+0.91NO2(g) +8H++7 e-NH4++ H2O0.90NO2-+8 H++6 e-NH4++2 H2O0.90ClO-+2 H++2 e-Cl-+ H2O0.89N2O4(g) +16 H++14 e-2NH4++4 H2O0.89NO3-+10 H++8 e-NH4++3 H2O0.875N2O4(g) +2 e-2NO2-0.87HNO2+6 H++6 e-NH3+2 H2O0.864SO3 2-+12 H++6 e-S4O62-+6 H2O0.86Hg2++2 e-Hg(l)0.85SnO32-+6 H++2 e-Sn2++3 H2O0.85NO(g) +6 H++5 e-NH4++ H2O0.84NO3-+2 H++2 e-NO2-+ H2O0.835O2+4 H++4 e-2 H2O0.815(Ph=7)NO2-+7 H++6 e-NH3(aq)+2 H2O0.812NO3-+2 H++2 e-N2O4(g)+2 H2O0.803Hg22++2 e-Hg(l)0.80Ag++ e-Ag(s)0.80NO2-+7 H++6 e-NH3(g)+2 H2O0.79NO3- (HNO3 75%) +2H++ e-NO2(g)+ H2O0.775Fe3++ e-Fe2+0.77PtCl4-+2 e-Pt(s)+4Cl-0.74HNO2+6 H++6 e-NH3(aq)+2 H2O0.732AsO4 3-+10 H++4 e-As2O3(s)+5 H2O0.72O2+2 H++2 e-H2O20.69ClO2-+ H2O +2 e-ClO-+2OH-0.68H2AsO4-+3 H++3 e-HAsO2+2 H2O0.67
Table des potentiels standard Ox+ne-↔Red à 25°C et à p=101kPaOxydantRéducteurE0 (Volt)2SO32-+6 H++4 e-S2O32-+3 H2O0.67Ag2SO4(s)+2 e-2Ag(s)+SO4 2-0.65Cu2++Br-+e-CuBr(s)0.65AsO43-+8H++5 e-As(s)+4 H2O0.65N2O(g)+10H++8 e-2NH4++ H2O0.65ClO3-+3 H2O +6 e-Cl-+6OH-0.62I2(aq)+2 e-2I-0.62Hg2SO4+2 e-2Hg(l)+SO42-0.61HAsO4-+3H++2 e-AsO2-+2 H2O0.61MnO4-+2 H2O +3 e-MnO2+4OH-0.60CH3OH+2 H++2 e-CH4(g)+ H2O0.592H3AsO4+4 H++4 e-AS2O3+5 H2O0.58BrO3-+3 H2O +6 e-Br-+6OH-0.584HSO3-+8 H++6 e-S4O62-+6 H2O0.58H3AsO4+2 H++2 e-H3AsO3+ H2O0.56MnO4-+ e-MnO42-0.56Cu2++Cl-+ e-CuCl0.54I2(s)+2 e-2I-0.54I3-+2 e-3I-0.54Cu++ e-Cu(s)0.52N2O(g)+8 H++8 e-2NH3+ H2O0.514H2SO3+4 H++6 e-S4O62-+6 H2O0.514SO2(g)+8 H++6 e-S4O62-+6 H2O0.51H2SO3+4 H++4 e-S(s)+3 H2O0.50S2O32-+6 H++4 e-2S(s)+3 H2O0.50BrO3-+2 H2O +4 e-BrO-+4OH-0.49ClO3-+2 H2O +4 e-ClO-+4OH-0.492CO32-+4 H++2 e-C2O42-+2 H2O0.48IO-+H2O+2 e-I-+2OH-0.47SO2(g)+4 H++4 e-S(s)+2 H2O0.452HSO3-+4 H++4 e-S2O32-+3 H2O0.452BrO-+2 H2O +2 e-Br2(l)+4OH-0.45As2O5+10 H++10 e-2As(s)+5 H2O0.432ClO-+2 H2O +2 e-Cl2(g)+4OH-0.422H2SO3+2 H++4 e-S2O32-+3 H2O0.40
Table des potentiels standard Ox+ne-↔Red à 25°C et à p=101kPaOxydantRéducteurE0 (Volt)O2+2 H2O +4 e-4 OH-0.402O2+2 H++2 e-O3(g)+ H2O0.38Bi2O3+6 H++6 e-Bi(s)+3 H2O0.38ClO4-+ H2O +2 e-ClO3-+2OH-0.37Sb(OH)6-+2 H++2 e-Sb(OH)4-+2 H2O0.36[Fe(CN)6]3-+ e-[Fe(CN)6]4-0.36MnO4-+4 H2O +5 e-Mn(OH)2+6OH-0.34Cu2++2 e-Cu(s)0.34Ag2O+ H2O +2 e-Ag(s)+2OH-0.34HSnO2 -+3 H++2 e-Sn(s)+2 H2O0.33Bi3++3 e-Bi(s)0.32CO32-+3 H++2 e-HCOO-+ H2O0.31As3++3 e-As(s)0.30ClO3-+ H2O +2 e-Clo2-+2OH-0.29MnO42-+ e-MnO43-0.27N2(g)+8 H++6 e-2NH4+0.27Hg2Cl2(s)+2 e-2Hg(l)+2Cl-0.27H2PO4-+9 H++8 e-PH3+4 H2O0.26IO3-+3 H2O +6 e-I-+6OH-0.26CO+6 H++6 e-CH4(g)+ H2O0.26PbO2+ H2O +2 e-PbO(r)0.25H3AsO3+3 H++3 e-As+3 H2O0.24As2O3+6 H++6 e-2As+3 H2O0.23AgCl+ e-Ag(s)+Cl-0.22HPO42-+10 H++8 e-PH3+4 H2O0.21CO2+4 H++4 e-C+2 H2O0.21CO32-+6 H++4 e-C+3 H2O0.21S(s)+2 H++3 e-H2S(g)0.17BiOCl+2 H++8 e-Bi(s)+Cl-+ H2O0.17CO2+8 H++8 e-CH4(g)+2 H2O0.17Co(OH)3+ e-Co(OH)2+OH-0.17SO42-+4 H++2 e-H2SO3+ H2O0.16Cu2++ e-Cu+0.16Sn4++2 e-Sn2+0.152NO2-+3 H2O +4 e-N2O(g)+6OH-0.15
Table des potentiels standard Ox+ne-↔Red à 25°C et à p=101kPaOxydantRéducteurE0 (Volt)S(s)+2 H++2 e-H2S(aq)0.14C+4 H++4 e-CH4(g)0.13CuCl+ e-Cu(s)+Cl-0.12NiO(s)+2 H++ e-Ni(s)+ H2O0.12SnO2+4 H++2 e-Sn2++ H2O0.12PO43-+11H++8 e-PH3+4 H2O0.12Sb3++3 e-Sb(s)0.10HgO(r)+ H2O +2 e-Hg(l)+2OH-0.098S4O62-+2 e-2S2O32-0.08AgBr+ e-Ag(s)+Br-0.071Bi(OH)2++3 e-Bi(s)+OH-0.07Sn4++4 e-Sn(s)0.05HOCN+2H++2 e-HCN(aq)+ H2O0.02NO3-+ H2O +2 e-NO2-+2OH-0.012 H++2 e-H2(g)0.00HOCN+2 H++2 e-HCN(g)+ H2O-0.02Fe3++3 e-Fe(s)-0.04P(bl)+3 H++3 e-PH3(g)-0.06N2+6 H++6 e-2NH3(g)-0.06O2+ H2O +2 e-OH-+HO2--0.065N2+6H2+6 e-2NH3(aq)-0.09CrO42-+4 H2O +3 e-Cr(OH)3+5OH--0.11P(r)+3 H++2 e-PH3(g)-0.11NO3-+6 H2O +8 e-NH3(g)+9OH--0.12PO43-+3 H++2 e-HPO32-+ H2O-0.12Pb2++2 e-Pb(s)-0.13Si+4 H++4 e-SiH4-0.14Sn2++2 e-Sn(s)-0.14OCN-+2 H++2 e-CN-+ H2O-0.14AgI+ e-Ag(s)+I--0.15HPO32-+8 H++6 e-PH3(g)+3 H2O-0.20As(s)+3 H++3 e-AsH3(g)-0.22HPO42-+2 H++2 e-HPO32-+ H2O-0.23CdS(s)+2 e-Cd(s)+S2--0.25Ni2++2 e-Ni(s)-0.25
Table des potentiels standard Ox+ne-↔Red à 25°C et à p=101kPaOxydantRéducteurE0 (Volt)H2PO3-+7 H++6 e-PH3(g)+3 H2O-0.26H2PO4- +2 H++2 e-H2PO3-+ H2O-0.26PbCl2+2 e-Pb(s)+2Cl--0.27Co2++2 e-Co(s)-0.28H3PO3+6 H++6 e-PH3(g)+3 H2O-0.28H3PO4+8 H++8 e-PH3(g)+4 H2O-0.28H3PO4+2 H++2 e-H3PO3+ H2O-0.28O2+ e-O2--0.28CuO+ H2O +2 e-Cu(s)+2OH--0.29H3PO4+H++2 e-H2PO3-+H2O-0.33Cd2++Hg+2 e-Cd(Hg)-0.35PbI2+2 e-Pb(s)+2I--0.36Cu2O(s)+ H2O +2 e-Cu(s)+2OH--0.36N2+6 H2O +6 e-2NH3+6 OH--0.40Cd2++2 e-Cd(s)-0.402H++2 e-H2-0.40 pH=7Cr3++ e-Cr2+-0.42Fe2++2 e-Fe(s)-0.44S(s)+2 e-S2--0.45Bi2O3(s)+3 H2O +6 e-2Bi+6 OH--0.45NO2-+ H2O + e-NO+2 OH--0.462CO2+2 H++2 e-H2C2O4-0.48ClO3-+ H2O + e-ClO2(g)+2 OH--0.48Sb+3 H++3 e-SbH3(g)-0.512NH4++2 e-2NH3(aq)+H2-0.55PbO(r)+ H2O +2 e-Pb+2 OH--0.582 SO32-+3 H2O +4 e-S2O32-+6 OH--0.58SO32-+3 H2O +6 e-S2++6 OH--0.61SbO2-+2 H2O +3 e-Sb+6 OH--0.64SO32-+3 H2O +4 e-S(s)+6 OH--0.66AsO2-+2 H2O +3 e-As(s)+4 OH--0.68Co(OH)2+2 e-Co(s)+2 OH--0.73S2O32-+3 H2O +4 e-2S+6 OH--0.74Cr3++3 e-Cr(s)-0.74
Table des potentiels standard Ox+ne-↔Red à 25°C et à p=101kPaOxydantRéducteurE0 (Volt)Zn2++2 e-Zn(s)-0.762 H2O +2 e-H2+2 OH--0.832NO3-+2 H2O +2 e-N2O4(g)+4 OH--0.86Cr2++2 e-Cr(s)-0.90HSnO2-+ H2O +2 e-Sn(s)+3 OH--0.91SO42-+ H2O +2 e-SO32++2 OH--0.94BF4-+3 e-B(s)+4F--1.04[Zn(NH3)4]2++2 e-Zn(s)+4NH3-1.04Sn(s)+4 H++4 e-SnH4-1.07PO43-+2 H2O +2 e-HPO32-+3 OH--1.12Mn2++2 e-Mn(s)-1.18As(s)+3 H2O +3 e-AsH3(g)+3 OH--1.37SiF62-+4 e-Si+6F--1.40ZnS+2 e-Zn(s)+S2--1.44Al3++3 e-Al(s)-1.67SiO32-+3H2O+4 e-Si(s)+6OH--1.70Be2++2 e-Be(s)-1.85H2+2 e-2H--2.25Al(OH)3+3 e-Al(s)+3OH--2.30Mg2++2 e-Mg(s)-2.36Mg(OH)2+2 e-Mg(s)+2OH--2.69Na++ e-Na(s)-2.71Ba(OH)2+2 e-Ba(s)+2OH--2.81Ca2++2 e-Ca(s)-2.84Sr(OH)2+2 e-Sr(s)+2OH--2.88Sr2++2 e-Sr(s)-2.89Ba2++2 e-Ba(s)-2.92Cs++ e-Cs(s)-2.92K++ e-K(s)-2.92Ca(OH)2+2 e-Ca(s)+2OH--3.03Li++ e-Li(s)-3.043N2+2 e-2N3--3.40
quotesdbs_dbs29.pdfusesText_35[PDF] claforan iv
[PDF] claforan dilution
[PDF] claforan posologie
[PDF] claforan per os
[PDF] claforan cp
[PDF] claforan dci
[PDF] claforan 1g
[PDF] claforan comprimé
[PDF] reglage chasse d'eau villeroy et boch
[PDF] regler chasse d'eau geberit
[PDF] la morte amoureuse résumé
[PDF] installer claroline connect en local
[PDF] claroline github
[PDF] installer claroline en local
