 Molarity and Normality
Molarity and Normality
Normality is always a multiple of molarity. It describes the “equivalent” moles of reactants involved in chemical reactions. An “equivalent” is an older
 TITRIMETRIC ANALYSIS
TITRIMETRIC ANALYSIS
is the molarity (M). Therefore N = a M … (vii). Equation (vii) is the expression for the relationship between normality and molarity. By using equation (vii)
 MOLARITY(M)MOLALITY (m)& NORMALITY(N).pdf
MOLARITY(M)MOLALITY (m)& NORMALITY(N).pdf
MOLALITY%20(m)&%20NORMALITY(N).pdf
 Unit 1 Units of measurements of solutes in solution e.g. normality
Unit 1 Units of measurements of solutes in solution e.g. normality
A standard solution contains accurately known concentration of solute. We can express the concentration in molality molarity
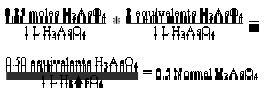 Molarity Molality and Normality
Molarity Molality and Normality
12-Jan-2006 Molarity is the number of moles of a solute dissolved in a liter of solution. A molar solution of sodium chloride is made by placing 1 mole of a ...
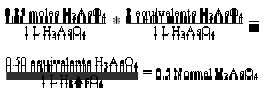 Molarity Molality and Normality
Molarity Molality and Normality
12-Jan-2006 Molarity is the number of moles of a solute dissolved in a liter of solution. A molar solution of sodium chloride is made by placing 1 mole of a ...
 Untitled
Untitled
➢Both Normality and Molarity are measures of concentration . ➢Molarity is a measure of the no. of moles per liter of solution . ➢Normality changes depending
 Concentrations of Common Commercial Acids and Bases
Concentrations of Common Commercial Acids and Bases
Gravity Molarity Normality. Reagent. Percent. (w/w). To Prepare. 1L of 1 Molar. Solution. Acetic Acid Glacial (CH3COOH) 60.05. 1.05. 17.4. 17.4. 99.7%. 57.5mL.
 UNITS OF CONCENTRATION.pdf
UNITS OF CONCENTRATION.pdf
Some of these are listed below. Molarity M = moles solute/liter of solution. Normality
 Molarity & Normality
Molarity & Normality
contains 0.25 mol of sodium hydroxide in every litre of solution. Page 25. Calculation of Molarity. To calculate the molarity of a solution
 [PDF] Molarity Molality and Normality
[PDF] Molarity Molality and Normality
12 jan 2006 · The first three: molality molarity and normality are dependant upon the mole unit The last two: percent by volume and percent by weight have
 [PDF] Both Normality and Molarity are
[PDF] Both Normality and Molarity are
?Both Normality and Molarity are measures of concentration ?Molarity is a measure of the no of moles per liter of solution
 [PDF] Some Basic Concepts of Chemistry
[PDF] Some Basic Concepts of Chemistry
Normality (N) It is the number of gram equivalent of solute present in one litre of solution : N V = Eq in litres ( ) (i) Molarity (M) It is the moles
 [PDF] MOLARITY(M)MOLALITY (m)& NORMALITY(N)pdf
[PDF] MOLARITY(M)MOLALITY (m)& NORMALITY(N)pdf
MOLALITY%2520(m)%26%2520NORMALITY(N).pdf
 [PDF] lectures-1-2pdf - Cal State LA
[PDF] lectures-1-2pdf - Cal State LA
Molarity (M) = # moles solute/#liters of solution The normality of permanganate ion is five times its molarity because MnO4 - ion accepts 5 electrons
 [PDF] 1)Solution: A homogenous mixture of two or more - VG Vaze College
[PDF] 1)Solution: A homogenous mixture of two or more - VG Vaze College
ii) Molarity iii) Molality iv) Mole fraction Ans: i) Normality: It is defined s the number of gram equivalents of the solute dissolved in one dm
 [PDF] Molarity Molality Normality and Mass Percent Worksheet IIpdf
[PDF] Molarity Molality Normality and Mass Percent Worksheet IIpdf
Molarity = Moles of solute / Liters of Solution (abbreviation = M) Molality = Moles of solute / Kg of Solvent (abbreviation = m) Normality = number of
[PDF] normality problems with solution pdf
[PDF] normering examen economie havo 2016
[PDF] northrop grumman das
[PDF] northrop grumman f 35 cni
[PDF] northstar listening and speaking pdf
[PDF] notation scientifique exercices corrigés 4eme
[PDF] notation scientifique seconde exercice corrigé
[PDF] note names in the bass sheet answers
[PDF] note values worksheet
[PDF] notes of c language
[PDF] notes of c language for bca
[PDF] notes of c language pdf
[PDF] notes of c language pdf download
[PDF] notes of c language pdf in hindi
