 Plan accès Villepinte - Hall 1.pdf
Plan accès Villepinte - Hall 1.pdf
Parc des Expositions de Villepinte. ACCES CANDIDATS. Parc des Expositions de Paris-Nord Villepinte - Hall 1. 93420 VILLEPINTE. Moyens d'accès: En RER: Ligne B.
 1_MTV EMA PARIS TICKET PURCHASE TERMS
1_MTV EMA PARIS TICKET PURCHASE TERMS
19 oct. 2023 5 novembre 2023 à Paris Nord Villepinte ZAC Paris Nord 2
 Informations sur lexposition Informations de lutilisateur THIVIN EVA
Informations sur lexposition Informations de lutilisateur THIVIN EVA
8 janv. 2022 PARIS - VILLEPINTE (93420) PARC DES EXPOSITIONS ZAC PARIS NORD 2 - 93420 VILLEPINTE. Groupe organisateur: SOCIETE CENTRALE CANINE - SCC.
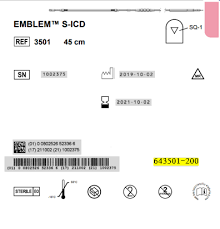 Electrode de défibrillation sous-cutanée Emblem™ S-ICD
Electrode de défibrillation sous-cutanée Emblem™ S-ICD
21 juil. 2021 f. Boston Scientific International S.A.. ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte ... produits2. Depuis la ...
 AU_AVT Functional Latching
AU_AVT Functional Latching
15 sept. 2022 f. Boston Scientific International S.A.. ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte ... 2— Partagez ces informations ...
 Bienvenue
Bienvenue
PARIS TERRES D'ENVOL. ADRESSE PHYSIQUE. 50 allée des impressionnistes. ZA Paris Nord 2. 93420 Villepinte. ADRESSE POSTALE. BP 10 018. 93601 Aulnay-sous-Bois.
 EXHIBITORS GUIDE
EXHIBITORS GUIDE
MILIPOL PARIS 2023. Parc des Expositions Paris Nord Villepinte. ZAC Paris Nord 2. 93420 Villepinte - France. MANDATORY INFORMATION: Exhibitor names and stand
 AU_AVT Functional Latching
AU_AVT Functional Latching
15 sept. 2022 f. Boston Scientific International S.A.. ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte ... 2— Partagez ces informations ...
 GUIDE DE LEXPOSANT
GUIDE DE LEXPOSANT
Parc des Expositions de Paris-Nord Villepinte – Festival Japan Expo. <Nom de votre Société>. <N° du stand et N° du Hall>. ZAC Paris-Nord 2. 93420 Villepinte.
 Duodenoscopio de un solo uso EXALT™ modelo D
Duodenoscopio de un solo uso EXALT™ modelo D
3 mar 2022 f. Boston Scientific International S.A.. ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte.
 AU_AVT Functional Latching
AU_AVT Functional Latching
2 jul 2021 f. Boston Scientific International S.A.. ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte.
 AU_AVT Functional Latching
AU_AVT Functional Latching
15 jul 2021 (RVTF) with Product List f. Boston Scientific International S.A.. ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte.
 Untitled
Untitled
12 jun 2017 2. Name and address of the manufacturer or his authorised representative: ... 22 Avenue des Nations ZAC Paris Nord II. 93420 Villepinte.
 EXHIBITORS GUIDE
EXHIBITORS GUIDE
Parc des Expositions Paris Nord Villepinte. ZAC Paris Nord 2. 93420 Villepinte - France. MANDATORY INFORMATION: Exhibitor names and stand number.
 Subject: Important Medical Device Advisory Update to December
Subject: Important Medical Device Advisory Update to December
6 jul 2021 f. Boston Scientific International S.A.. ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte.
 Zurpaz™ Steerable Sheath 8.5F Asymmetric and Symmetric Curve
Zurpaz™ Steerable Sheath 8.5F Asymmetric and Symmetric Curve
ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte. Siège social : Parc du Val Saint Quentin – 2 rue René Caudron.
 Subject: Important Medical Device Advisory Update to December
Subject: Important Medical Device Advisory Update to December
5 jul 2021 f. Boston Scientific International S.A.. ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte.
 Cover letter Urgent Field Safety Notices:
Cover letter Urgent Field Safety Notices:
25 jun 2021 f. Boston Scientific International S.A.. ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte.
 Eluvia 150mm and Innova 180mm & 200mm Stent Systems
Eluvia 150mm and Innova 180mm & 200mm Stent Systems
ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses – 93420 Villepinte. Siège social : Parc du Val Saint Quentin – 2 rue René Caudron.
Boston Scientific International S.A.
ZAC Paris Nord II/Bât Emerson - 33 rue des Vanesses 93420 Villepinte Siège social : Parc du Val Saint Quentin 2 rue René Caudron78960 Voisins le Bretonneux France
Tel 33 (0)1 48 17 47 00
Fax 33 (0)1 48 17 47 01
www.bostonscientific.com Adresse postale : BP 53343 Villepinte 95941 Roissy Charles de Gaulle France- RCS Bobigny 420 668 402 0 0035 Code APE 8559 A Page 1 of 3
"Hospital_Name» "Users_Name»- "Department» "Customer_Address» "Zip_Code» "City» -"Country_name»Reference: 92169170-FA xx November 2017
-8UJHQW0HGLFDO'HYLFH5HFDOOEluviaTM
150mm and
InnovaTM 180mm & 200mm Stent Systems
Dear "Users_Name»,
Boston Scientific is initiating a voluntary removal of the 150mm EluviaTM Drug-Eluting Vascular Stent System and the 180mm and 200mm InnovaTM Self-Expanding Stent System due to elevated complaint rates for partial stent deployment. No other EluviaTM or InnovaTM stent system sizes are included in this voluntary removal. In addition, this action does not include previously implanted devices.Partial deployment occurs when the stent is unable to be fully released from the delivery system. Part
of the stent can become anchored in the vessel while the rest of the stent remains within the delivery system. The most common reported injury has been additional medical or minor surgical
intervention, vessel trauma or prolongation of the implant procedure. However, in certain cases Boston Scientific has received reports of major surgery to retrieve the stent/delivery system or to correct vascular compromise. Our records indicate that your facility received some of the concerned product.The table below (Attachment 1) provides a complete list of all affected products, including
Product Description and Material Number (UPN). Please note that only the devices listed below are affected. No other Boston Scientific product is involved in this Field Safety Notice. Further distribution or use of any remaining affected product should cease immediately.Page 2 of 3
INSTRUCTIONS:
1- Please immediately discontinue use of the Boston Scientific product reported in the list and
remove all of the affected units from your inventory, regardless of where these units are stored in your facility. Segregate the units in a secure place, pending return to Boston Scientific. 2 - Please complete the attached Verification Form even if you do not have any product to return. 3 - When completed, please return the Verification Form to your local Boston Scientific office for the attention of "Customer_Service_Fax_Number» on or before XXNovember 2017.
4 - If you have products to return, please package them in an appropriate shipping box and contact "Customer_Service_Tel» of your local Boston Scientific office, to arrange return. 5 - Please pass this notice to any healthcare professional from your organization that needs to beaware and to any organization where the potentially affected devices have been transferred (If
appropriate). Please provide Boston Scientific with details of any affected devices that have been transferred to other organizations (if appropriate).Your Competent Authority is
being notified of this Field Safety Notice. We regret any inconvenience that this action may cause, and we appreciate your understanding as we act to ensure patient safety and customer satisfaction.If you have any questions or would like assistance with this Field Safety Notice, please contact your
local Sales Representative. Yours sincerely, Attachment: Verification Form Boston Scientific International S.A.Page 3 of 3
Attachment 1 : Affected Product Listing
EluviaTM 150mm and InnovaTM 180mm & 200mm Stent Systems listed below are affectedInnova 180mm, 200mm - EU
Product Description Material Number
(UPN)Innova EU 5x180x75 H74939180051870
Innova EU 5x200x75 H74939180052070
Innova EU 6x180x75 H74939180061870
Innova EU 6x200x75 H74939180062070
Innova EU 7x180x75 H74939180071870
Innova EU 7x200x75 H74939180072070
Innova EU 8x180x75 H74939180081870
Innova EU 8x200x75 H74939180082070
Innova EU 5x180x130 H74939181051830
Innova EU 5x200x130 H74939181052030
Innova EU 6x180x130 H74939181061830
Innova EU 6x200x130 H74939181062030
Innova EU 7x180x130 H74939181071830
Innova EU 7x200x130 H74939181072030
Innova EU 8x180x130 H74939181081830
Innova EU 8x200x130 H74939181082030
Eluvia 150mm- OUS
Product Description Material Number
(UPN)EU DES SFA, 6X150, 75 cm H74939295601570
EU DES SFA, 6X150, 130 cm H74939295601510
EU DES SFA, 7X150, 75 cm H74939295701570
EU DES SFA, 7X150, 130 cm H74939295701510
quotesdbs_dbs14.pdfusesText_20[PDF] paris öffentliche verkehrsmittel karte
[PDF] paris öffentliche verkehrsmittel plan pdf
[PDF] paris one day metro pass cost
[PDF] paris orly geneve avion easyjet
[PDF] paris ouest automobile 59 rue de pontoise 95870 bezons
[PDF] paris ouest automobile bezons horaires
[PDF] paris ouest la defense nanterre
[PDF] paris ouest la defense siret
[PDF] paris outragé paris martyrisé
[PDF] paris outragé paris martyrisé figure de style
[PDF] paris peace conference
[PDF] paris peace conference notes
[PDF] paris peace conference summary
[PDF] paris peace summit
