 Chemistry 209 Expt 5 - Esterification
Chemistry 209 Expt 5 - Esterification
This reaction termed Fischer esterification in honor of its discoverer
 The ester is synthesised via a Fischer esterification – the reaction of
The ester is synthesised via a Fischer esterification – the reaction of
- Employing a carboxylic acid derivative such as an acid chloride
 EXPERIMENT 5 SYNTHESIS OF ESTERS USING ACETIC
EXPERIMENT 5 SYNTHESIS OF ESTERS USING ACETIC
This reaction termed Fischer esterification in honor of its discoverer
 Experiment 14A: Isopentyl Acetate
Experiment 14A: Isopentyl Acetate
Oct 14 2020 Theoretical Yield: 8.85 mmol. isopentyl acetate may be produced. ... focused on the synthesis of an ester by a Fischer esterification.
 EXPERIMENT 5 ORGANIC SYNTHESIS: FISCHER
EXPERIMENT 5 ORGANIC SYNTHESIS: FISCHER
In this experiment you will synthesize the ester n-butyl acetate (bp 126.5°C)
 Fischer Esterification
Fischer Esterification
Loss of water yields a carbocation stabilized by resonance which need only lose a proton to give the desired ester
 Improved Fischer Esterification of Substituted Benzoic Acid under
Improved Fischer Esterification of Substituted Benzoic Acid under
alcohols produced the highest yield of the ester product compared to secondary alcohols and tertiary alcohol resulted in the lowest.
 Multistep Synthesis Ester step
Multistep Synthesis Ester step
Step 3: Fischer Esterification of m-Nitrobenzoic Acid to Produce Methyl m- To do so you can use the percent yields for each step to compute the overall.
 Synthesis and Investigation of Thermal Properties of Highly Pure
Synthesis and Investigation of Thermal Properties of Highly Pure
Jun 30 2018 Carboxylic esters can be obtained via Fischer esterification of carboxylic acids with alcohols
 Fischer Esterification of 3-ntrobenzoic acid 2017
Fischer Esterification of 3-ntrobenzoic acid 2017
Fischer Esterification of 3-nitrobenzoic acid to Produce Methyl 3- To do so you can use the percent yields for each step to compute the overall.
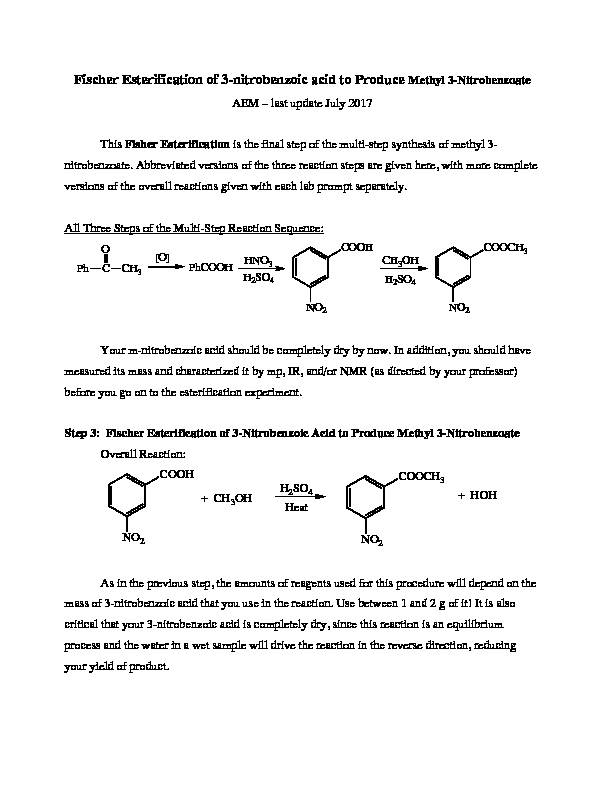
Fischer Esterification of 3-nitrobenzoic acid to Produce Methyl 3-Nitrobenzoate AEM - last update July 2017 This Fisher Esterification is the final step of the multi-step synthesis of methyl 3-nitrobenzoate. Abbreviated versions of the three reaction steps are given here, with more complete versions of the overall reactions given with each lab prompt separately. All Three Steps of the Multi-Step Reaction Sequence: Your m-nitrobenzoic acid should be completely dry by now. In addition, you should have measured its mass and characterized it by mp, IR, and/or NMR (as directed by your professor) before you go on to the esterification experiment. Step 3: Fischer Esterification of 3-Nitrobenzoic Acid to Produce Methyl 3-Nitrobenzoate Overall Reaction: As in the previous step, the amounts of reagents used for this procedure will depend on the mass of 3-nitrobenzoic acid that you use in the reaction. Use between 1 and of it! It is also critical that your 3-nitrobenzoic acid is completely dry, since this reaction is an equilibrium process and the water in a wet sample will drive the reaction in the reverse direction, reducing your yield of product. PhCCH
3 O [O]PhCOOH
HNO 3 H 2 SO 4 COOH NO 2 CH 3 OH H 2 SO 4 COOCH 3 NO 2 COOH NO 2 + CH 3 OH H 2 SO 4 COOCH 3 NO 2 Heat + HOHREACTION: For each g of 3-nitrobenzoic acid you will need 8 mL of anhydrous methanol; for each 20 mL of methanol, you will need 1 mL of concentrated H2SO4. Note that this concentrated acid should be measured using your graduated cylinder for safe handling, even if that requires some estimation! Consider the total volume of this mixture, and choose a roundbottom flask that holds about twice that volume; in other words, choose a flask so that it is about half full. Put the three materials in the proportions described above into the roundbottom flask with a couple of boiling chips, and attach a reflux condenser to form a reflux apparatus. Heat to reflux for 1 hour. WORK-UP: When the roundbottom flask has cooled enough to hold, pour the reaction mixture into a beaker of ice (use an ice volume of about 5 times the volume of methanol used) and stir. Use suction filtration to isolate the product and wash with water; the ice should melt during this process, but you can rinse it off and remove it if needed. The crude product should be recrystallized from methanol. Once it is completely dry, determine its mass, and calculate its theoretical and percent yield. Also, determine its purity by mp, IR, and/or NMR (as preferred by your professor). CLEAN UP. Combine all the solutions, check the pH, neutralize as needed, and flush down the drain with lots of water. Solid methyl 3-nitrobenzoic acid should be placed in the solid hazardous waste. Multi-Step Synthesis Yield Calculation. When you carry out a series of reaction steps, you usually want to know the efficiency of the whole process. To do so, you can use the percent yields for each step to compute the overall percent yield. This is easiest to explain with an example. Suppose you carried out four reactions in sequence with the percent yields given below. Step 1: 87.5%; Step 2: 91.2%; Step 3: 79.3%; and Step 4: 81.9% The overall percent yield is computed as shown, here. Overall Percent Yield: 0.875 x 0.912 x 0.793 x 0.819 x 100 = 51.8% overall. Be sure to compute the overall percent yield for the three steps of you synthesis of methyl 3-nitrobenzoate.
quotesdbs_dbs2.pdfusesText_3[PDF] percentage of guns used in crimes
[PDF] perfect font size for a4 paper
[PDF] perfect pied a terre paris
[PDF] perfect size array java
[PDF] perfume formulas
[PDF] perfume maturation process
[PDF] perfume recipe book
[PDF] periodic table
[PDF] periodic table in minecraft
[PDF] périodicité de calcul de la valeur liquidative
[PDF] perma
[PDF] perma ™ theory of well being and perma ™ workshops
[PDF] permission to travel letter sample
[PDF] personal cell phone for business use policy
