 Chapter 5 - Colligative Properties
Chapter 5 - Colligative Properties
The result is often derived in physical chemistry books. In applying boiling point elevation to polymer solutions we should realize that polymer solu- tions
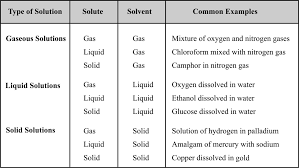 SOLUTIONS & COLLIGATIVE PROPERTIES
SOLUTIONS & COLLIGATIVE PROPERTIES
This phenomenon is called reverse osmosis. Application : Desalination of sea water : When pressure more than osmotic pressure is applied pure water is squeezed
 Online Application of Colligative Properties Solutions Experiment
Online Application of Colligative Properties Solutions Experiment
Abstract. This study aimed to describe the practical implementation of colligative properties by Online. Method used in this study was pre-experiment.
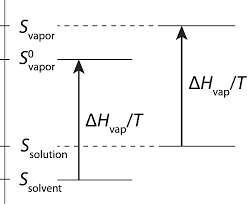 Colligative properties CH102 General Chemistry Spring 2014
Colligative properties CH102 General Chemistry Spring 2014
An important application of osmotic pressure is in the determination of molar masses of large molecules such as proteins. The reason is that usually
 Solutions
Solutions
Concentration described by mass percentage is commonly used in industrial chemical applications. colligative properties (colligative: from Latin: co means ...
 Multiple representation-based mobile apps with learning cycle 7e
Multiple representation-based mobile apps with learning cycle 7e
application of colligative properties of solutions that students have pdf. Page 12. “Multiple representation-based mobile apps with learning cycle 7e model ...
 REACT Strategy: Efforts to Link Concept Colligative Properties
REACT Strategy: Efforts to Link Concept Colligative Properties
Applying the concept of colligative properties of solutions is closely related to everyday life and science development so the REACT strategy can be applied in
 Diapositiva 1
Diapositiva 1
Definition of Colligative Property. • Vapour Pressure Lowering. • Freezing Point Depression (Cryoscopy). • Boiling Point Elevation (Ebullioscopy).
 How to develop colligative properties of solution chemistry e- book
How to develop colligative properties of solution chemistry e- book
Today school facilities have supported the implementation of digital-based learning. However
 International Journal of Instruction
International Journal of Instruction
5 Jun 2020 They were all not provided with chemical equipment and application of the Colligative Properties of. Solutions such as the sub-topic of ...
 Colligative Properties
Colligative Properties
colligative properties to measure the molecular weight of polymers. In applying boiling point elevation to polymer solutions we should realize that ...
 A Lecture on Colligative Properties in an Undergraduate Curriculum
A Lecture on Colligative Properties in an Undergraduate Curriculum
The effect of a solute on the vapor pressure may be determined in dilute solutions by applying the Raoult's Law (Eq. 1). o a. a a p = p x . (Eq.1).
 Colligative properties CH102 General Chemistry Spring 2014
Colligative properties CH102 General Chemistry Spring 2014
There are four colligative properties. • vapor-pressure lowering. • boiling-point elevation. • freezing-point depression. • osmotic pressure. Each of
 Formulas for Colligative Properties
Formulas for Colligative Properties
Formulas for Colligative Properties. Lowering of. Vapor Pressure. Elevating the. BOILING Point. Depression of the. FREEZING Point. Osmotic. Pressure.
 COLLIGATIVE-PROPERTIES.pdf
COLLIGATIVE-PROPERTIES.pdf
Application of Colligative properties (i) Explain the term Colligative property. (ii) State four Colligative properties of solution.
 Untitled
Untitled
Colligative Properties of. Electrolytes. •. Solution Dosage Forms. •. Application of Colligative. Properties. II. Colligative Properties of. Solutions.
 Developing Innovative Chemistry Laboratory Workbook Integrated
Developing Innovative Chemistry Laboratory Workbook Integrated
of Colligative Properties of solutions. The implementation of InoChemLaW was carried out onto the experimental class compared to the existing laboratory.
 Colligative Properties of Foods
Colligative Properties of Foods
Colligative Properties. 3.1. Depression of the Freezing Point. 3.1.1. Basic Concepts. 3.1.2 Applications to Foods. 3.2. Elevation of the Boiling Point.
 WORKSHEET:SOLUTIONS AND COLLIGATIVE PROPERTIES SET
WORKSHEET:SOLUTIONS AND COLLIGATIVE PROPERTIES SET
WORKSHEET:SOLUTIONS AND COLLIGATIVE PROPERTIES. SET A: 1. Find the molarity of all ions in a solution that contains 0.165 moles of aluminum chloride in 820.
 Lecture 4: Colligative Properties
Lecture 4: Colligative Properties
By definition a colligative property is a solution property (a property of mixtures) for which it is the amount of solute dissolved in the solvent matters
[PDF] application of derivatives in daily life pdf
[PDF] application of derivatives in physics pdf
[PDF] application of derivatives pdf
[PDF] application of derivatives pdf target
[PDF] application of derivatives ppt
[PDF] application of derivatives problems with answers pdf
[PDF] application of e learning in education
[PDF] application of fermentation
[PDF] application of fermentation in food industry
[PDF] application of fir filter in medical
[PDF] application of gps
[PDF] application of laplace transform
[PDF] application of laplace transform to boundary value problems
[PDF] application of laplace transform to differential equations calculator
