 MODULE 5: DISTILLATION
MODULE 5: DISTILLATION
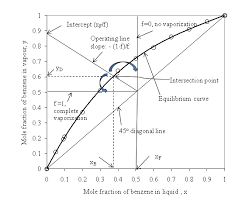
The Figure 5.1 shows a constant pressure phase diagram for an ideal solution. (one that obeys Raoult's Law). At constant pressure depending on relative. Page
 DETERMINATION OF PROLINE IN MIXTURES CONTAINING l-AND
DETERMINATION OF PROLINE IN MIXTURES CONTAINING l-AND
e hodanila$e. III ppt. per cent. 97.8. 93.9. 88.7. 74.4. 10.6 l Meyer (2) has found that this simple equation which follows from Raoult's law
 STUDENTS SUPPORT MATERIAL CLASS-XII CHEMISTRY
STUDENTS SUPPORT MATERIAL CLASS-XII CHEMISTRY
State the following with one application of each: Henry law and Reverse osmosis. State Raoult's law for a solution containing a non volatile solute. Also ...
 Class - XII Multiple Choice Question Bank [MCQ ] Term – I & Term-II
Class - XII Multiple Choice Question Bank [MCQ ] Term – I & Term-II
Redox reactions EMF of a cell
 Properties of Solutions
Properties of Solutions
If the mole fraction of heptane is 0.600 what is the composition of the vapor above the solution? Strategy: DOWNSTAIRS use Raoult's law to find vapor pressure
 IB - 1 to 28 - FINAL
IB - 1 to 28 - FINAL
Please shade your mode of examination in the appropriate box PPT or CBT. (A) Point No. 1 of the Application Form: (NAME OF THE CANDIDATE). 19.4. Candidate
 chemistry/xii-(2020-21)
chemistry/xii-(2020-21)
APPLICATION OF HENRY'S LAW. 1. to increase the solubility of carbon dioxide in What is the similarity between Raoult's law and Henry law. Ans. The partial ...
 An Introduction To Headspace Sampling In Gas Chromatography
An Introduction To Headspace Sampling In Gas Chromatography
Raoult's Law states that the vapor pressure of a compound above a solution Figure 22 shows the result of using a ZDL for a typical HS application.
 SCHOOL NAME: WORKSHEET – 7/Module-7(OVERALL) Sub
SCHOOL NAME: WORKSHEET – 7/Module-7(OVERALL) Sub
higher boiling point. 7. Liquids A and B on mixing produce a warm solution. Which type of deviation from. Raoult's law is shown? 8
 LECTURE NOTES
LECTURE NOTES
Dilute Solutions: Vapour Pressure Raoult's Law
 Raoults Law and Its Application to Sublimation Vapor Pressures of
Raoults Law and Its Application to Sublimation Vapor Pressures of
Raoult's Law and Its Application to Sublimation Vapor Pressures of Mixtures of Polycyclic Aromatic Hydrocarbons. Jillian L. Goldfarb and Eric M. Suuberg*.
 DISTRIBUTION LAW (LIMITATIONS AND APPLICATIONS)
DISTRIBUTION LAW (LIMITATIONS AND APPLICATIONS)
APPLICATION OF DISTRIBUTION LAW. ? There are numerous applications of distribution law in the laboratory as well as in industry. (1) Solvent Extraction-.
 PHYSICAL PHARMACY
PHYSICAL PHARMACY
Ideal Solutions and Raoult's Law. In an ideal solution of two volatile liquids the partial vapor pressure of each volatile constituent is equal to the
 (Microsoft PowerPoint
(Microsoft PowerPoint
Dew point & Bubble point calculation with Raoult's Law. Bubble P : calculate (y Application of Raoult's law to species i requires a value for at the.
 VaporILiquid Equilibrium: Introduction
VaporILiquid Equilibrium: Introduction
Thus the liquid water is regarded as pure and Raoult's law for the water (species 2) becomes y2 P = Ppt. At 298.15 K (25'C) and atmospheric pressure
 GUSTAVO V. BARBOSA-CÁNOVAS
GUSTAVO V. BARBOSA-CÁNOVAS
The application of Raoult's Law to food systems is not practical because of solvent-solute interactions. Page 10. „ Assumes water activity lowering due to.
 Applications of Gas Laws in Anaesthesia delivery system- from
Applications of Gas Laws in Anaesthesia delivery system- from
Raoult's law. Definitions of the gas laws. 1)Boyle's Law: states that at constant temperature(T)the volume(V) of a given mass of a.
 An Introduction To Headspace Sampling In Gas Chromatography
An Introduction To Headspace Sampling In Gas Chromatography
To appreciate the principle let's consider an application that is these deviations from the ideal
 Estimation dénergies de GIBBS de solvatation pour les modèles
Estimation dénergies de GIBBS de solvatation pour les modèles
14 janv. 2020 Article : “Application of the corresponding-state law to the ... RAOULT Grandeur écrite en prenant comme référence un mélange de RAOULT.
 Introduction to Thermodynamics models for process engineering
Introduction to Thermodynamics models for process engineering
Raoult's law case of solvent. (Raoult's law) solute fugacity. Henry's law f1 pure f2 pur f2 ref=f2 pure symmetric reference unsymmetric reference.
ENVIRONMENTAL ENGINEERING SCIENCE
Volume 25, Number 10, 2008
©Mary Ann Liebert, Inc.
DOI: 10.1089/ees.2007.0195
Raoult's Law and Its Application to Sublimation Vapor Pressures of Mixtures of Polycyclic Aromatic HydrocarbonsJillian L. Goldfarb and Eric M. Suuberg
Division of Engineering, Brown University, Providence, RI. Received date: July 25, 2007Accepted date after revision: February 18, 2008Abstract
Polycyclic aromatic hydrocarbons (PAH) are ubiquitous pollutants resulting from incomplete combustion and
many fuel processing and storage operations. They are encountered in the environment in many forms, rang-
ing from non-aqueous phase liquid and/or solid mixtures (i.e., NAPL and NAPS materials), to dissolved aque-
ous phase components, to adsorbed species on solid surfaces. The present work concerns the vapor pressure
behavior of non-aqueous phase mixtures of PAH, as may characterize tars or solid PAH phase contaminants.
In particular, model mixtures of anthracene, perylene, and fluoranthene are examined herein. Mixture vapor
pressure data are important to understanding and modeling exposure opportunities. There exist few data in
the literature concerning the vapor-liquid and vapor-solid equilibrium behavior of mixtures of high molecular
weight organic compounds as they tend to degrade at the high temperatures required to conveniently perform
such measurements. This degradation issue is overcome by implementing the Knudsen effusion technique, an
indirect method of vapor pressure measurement at temperatures sufficiently low to ensure thermal stability of
the PAH studied. Our results indicate that commonly employed Raoult's law assumptions may represent an
over-simplification of some actual near-ambient PAH mixture behavior for the compounds studied, yet there
are many instances in which this law is closely followed.Key words:polycyclic aromatic hydrocarbons; PAH; perylene; fluoranthene; anthracene; vapor pressure; mix-
ture; Knudsen effusion; Raoult's law 1Introduction
VAPORPRESSUREis a fundamental property key to pre-
dicting and modeling the fate and transport of a com- pound or mixture within the environment. There are many common environmental pollutants, such as polycyclic aro- matic hydrocarbons (PAH), for which there exist few, if any, literature data on their vapor pressures. In recent years, sev- eral laboratories have presented new pure PAH vapor pres- sure data (Chickos et al.,1998; Goldfarb and Suuberg, 2008; Ribeiro da Silva et al.,2006; Ruzicka et al.,2005; Verevkin,2003). Vapor pressure is highly dependent on the intra- and
intermolecular interactions of the chemical species of inter- est. As such, vapor pressures may vary by many orders of magnitude for organic compounds of comparable molecular size. Reported vapor pressures of common PAH range from 10 -2 to 10 -12 atmospheres at ambient conditions. Despite the fact that the vapor pressures of some PAH are 12 orders of magnitude less than atmospheric pressure, vapor pressures may still play a pivotal role in environmental transport (Schwarzenbach et al., 1993). Measurements on pure compound vapor pressures repre- sent only part of the necessary data for understanding fate and transport. More often than not, PAH are present as mix- tures within the environment, often in contact with other phases. At former manufactured gas plants (MGPs), soils may contain tars - dense nonaqueous phase liquids (DNAPLs) or nonaqueous phase solids (NAPS) that are mixtures of hun- dreds, even thousands of different PAH. A dearth of mix- ture thermodynamic data, including vapor pressure, can hin- der the modeling of volatilization and the fate and transport of mixtures within the environment (Voutsas et al.,2005;Weschler, 2003).
There are many relationships that describe the thermody- namic behavior of multi-phase and multi-component mix- tures. One of the simplest and most widely applied for non- aqueous mixtures is Raoult's law. It is used to estimate the contribution of individual components of a liquid or solid mixture to the total pressure exerted by the system, espe- cially for discrete mixtures where the quantity of each com- ponent is known. *Corresponding author:Division of Engineering, Brown University, Providence, RI. Phone:401-863-1420; Fax:401-863-9120; E-mail:Eric_Suuberg@brown.edu
Ideal solutions are mixtures that obey Raoult's law through the entire range of compositions of the mixture. P vap i x i P i°(1)
The vapor pressure of the mixture, P
vap , is equal to the sum of x i , the components' mole fractions, times P i°, their re-
spective pure component vapor pressures. While commonly applied to liquid mixtures, Raoult's law is also applied to solid mixtures undergoing sublimation. Some mixtures, especially those of chemically similar com- ponents (i.e., similar polarity, structure, aromatic nature, and hetero-atoms present), obey Raoult's law quite well. Sub- stances with uniform intermolecular forces are most able to fully obey Raoult's law. Woodrow (2003) reported that the fuel vapor of a jet fuel mixture behaved according to Raoult's law predictions. Despite this being a mixture of heavy hy- drocarbons, the generally uniform chemical nature of the jet fuel components contributes to the observed ideal behavior (Woodrow, 2003). On the other hand, as early as 1934, Beatty and Calingaert (1934) observed that hydrocarbon systems could experience non-ideality when aromatic hydrocarbons are present. Like- wise, Djordjevic (1991) found that mixtures of several poly- cyclic aromatic hydrocarbons in n-octadecane are slightly non-ideal, attributed to the compensation between enthalpic and entropic effects. Activity coefficients determined by Aoulmi et al. (1995) for mixtures of PAH in long-chain alka- nes ranged from 0.37 for naphthalene in squalane to 3.86 for anthracene in n-C 36, with many of the mixtures having ac- tivity coefficients close to one, indicating only mildly non- ideal behavior. Along similar lines, several groups have stud- ied the partitioning behavior of PAH components between aqueous phases and nonaqueous phases, including Mukherji et al.(1997). Their work concluded that Raoult's law is a good approximation for non-aqueous phase behavior.
A study by Burks and Harmon (2001) examined vapor
phase concentrations of PAH in equilibrium with the solid state of pure PAH binary mixtures and with two lampblack- contaminated soils from former MGP sites (examples of NAPS). Two binary mixtures were investigated: fluorene- naphthalene (two compounds of widely different crystalline structures) and anthracene-naphthalene (of similar crystalGOLDFARB AND SUUBERG2
TABLE1.M IXTURESOFPUREPAH PREPAREDUSINGQUENCH-COOLMETHODMolecularMeltMin.
CAS Reg.WeightPointPurity Mole
MixtureCompoundFormula No.g/molC Supplier%Fraction1AnthraceneC
14 H 10120-12-7178.23217 Aldrich990.50
PeryleneC
20 H 12198-55-0252.31274 Aldrich99 0.50
2AnthraceneC
14 H 10120-12-7178.23217 Aldrich990.51
FluorantheneC
16 H 10206-44-0202.26110 TCI America 980.49
3AnthraceneC
14 H 10120-12-7178.23217 Aldrich990.50
PhenanthreneC
14 H 1085-01-8178.23100 Kodak, Inc 990.50
4PhenanthreneC
14 H 1085-01-8178.23100 Kodak, Inc 990.33
AnthraceneC
14 H 10120-12-7178.23217 Aldrich990.33
FluorantheneC
16 H 10206-44-0202.26110 TCI America 980.33
5PeryleneC
20 H 12198-55-0252.31274 Aldrich990.33
AnthraceneC
14 H 10120-12-7178.23217 Aldrich99 0.33
FluorantheneC
16 H 10206-44-0202.26110 TCI America 980.33
class with cell dimensions within 10% of each other). The au- thors found that the vapor pressures of both of these mix- tures were closer to the more volatile component present, as opposed to following a Raoult's law prediction. In addition, PAH behavior in the presence of soil core samples from two different former MGP sites (one a moist, fine to medium grain sand, and the other a wet, silt-clay) was studied. The authors concluded that independent PAH behavior occurs in some contaminated soil as they noted that the vapor pres- sures above the organic material-contaminated soils mimic- ked the results of the model PAH mixtures relative to their solid-phase concentrations. In these contaminated soils, the PAH achieved high partial pressures relative to their mea- sured solid-phase concentrations, despite the presence of other organics in the soil. This again calls into question the ability to describe such behavior using an ideal mixing model (though Burks and Harmon did not quantify the other or- ganics present, so a more formal analysis of the results is not possible). Another study by Oja and Suuberg (2005) reported the va- por pressures of model mixtures of solid PAH. For an equimolar mixture of anthracene and perylene, they noted no significant deviations from ideality. However, for a mix- ture of 25 mol% anthracene, balance benzofluorene, signifi- cant deviations from ideality occurred; at low temperatures the mixture vapor pressure behavior was that of two sepa- rate solid phases, but approached ideal behavior at high tem- peratures (but still well below the melting point of the mix- ture). This trend was noted for several other mixtures, suggesting, "that as the systems approach melting they be- gin to behave as more nearly ideal single phases" (Oja and Suuberg, 2005). When two components in two phases act in- dependently, the vapor pressure of that mixture is the sum- mation of the vapor pressure of the components at a given temperature, very different behavior than that predicted byEquation (1).
Oja and Suuberg saw very different behavior when the compounds comprising the mixture were chosen to promote interactions with one another. The vapor pressure of a nearly equimolar mixture of 1-hydroxypyrene and phenanthridine was far less than that predicted by Raoult's law. Such devi- ations were attributed to the strong interactions between the basic nitrogen of the phenanthridine ring and the acidic phe- T ABLE 2.SUMMARYOF
RESULTSOF
PAH MIXTURES
; CLAUSIUS
-CLAPEYRON
EQUATION
CONSTANTSWITH
95% CONFIDENCE
INTERVALAND
V APOR PRESSURE
EXTRAPOLATIONAT
298 KTemp % Error
MoleMelt Point, Range,
sub H sub H P vap Pquotesdbs_dbs10.pdfusesText_16[PDF] application of regular expression in compiler design
[PDF] application of regular expression in lexical analysis
[PDF] application of regular expression in python
[PDF] application of regular expression in tcs
[PDF] application of regular expression in theory of computation
[PDF] application of robots pdf
[PDF] application of satellite weather
[PDF] application of spectroscopy pdf
[PDF] application of supervised learning
[PDF] application of time value of money pdf
[PDF] application of vapour pressure
[PDF] application of word processing
[PDF] application of z transform in digital filters
[PDF] application of z transform in electrical engineering
