 IMDRF/SaMD WG/N12FINAL:2014
IMDRF/SaMD WG/N12FINAL:2014
18 sep 2014 This document was produced by the International Medical Device Regulators Forum. There are no restrictions on the reproduction or use of ...
 Software as a Medical Device (SaMD): Key definitions
Software as a Medical Device (SaMD): Key definitions
9 dec 2013 The document herein was produced by the International Medical Device Regulators Forum. (IMDRF) a voluntary group of medical device regulators ...
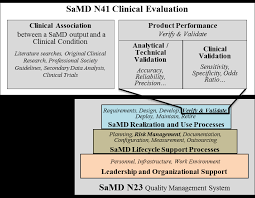 Final Document: Software as a Medical Device (SaMD): Clinical
Final Document: Software as a Medical Device (SaMD): Clinical
21 sep 2017 The International Medical Device Regulators Forum (IMDRF) seeks to establish a common and converged understanding of clinical evaluation and ...
 GHTF SG3 - Quality management system –Medical Devices
GHTF SG3 - Quality management system –Medical Devices
The document herein was produced by the Global Harmonization Task Force which is comprised of representatives from medical device regulatory agencies and
 IMDRF Strategic Plan 2021 - 2025
IMDRF Strategic Plan 2021 - 2025
25 sep 2020 The mission1 of the International Medical Device Regulators Forum (IMDRF) is to strategically accelerate international medical device regulatory ...
 imdrf pmd wg/n49 final: 2018
imdrf pmd wg/n49 final: 2018
18 okt 2018 The document herein was produced by the International Medical Device Regulators Forum. (IMDRF) a voluntary group of medical device regulators ...
 IMDRF Standard Operating Procedures
IMDRF Standard Operating Procedures
19 dec 2022 As an Affiliate Member the regulatory authority will participate in IMDRF by attending “open” meetings and using IMDRF documents in part or in ...
 UDI guidance: Unique Device Identification (UDI) of medical devices
UDI guidance: Unique Device Identification (UDI) of medical devices
9 dec 2013 This document was produced by the International Medical Device Regulators Forum. There are no restrictions on the reproduction or use of ...
 IMDRF/SaMD WG/N23 FINAL: 2015 - Software as a Medical Device
IMDRF/SaMD WG/N23 FINAL: 2015 - Software as a Medical Device
2 okt 2015 The IMDRF/SaMD WG/N12 document also highlights the use of quality management as a general consideration towards the safety effectiveness
 IMDRF: Methodological Principles in the Use of International
IMDRF: Methodological Principles in the Use of International
16 mrt 2017 This methodological document also builds on the IMDRF Common Data Elements (CDE) for Medical Device Identification document. The CDE effort ...
 IMDRF/SaMD WG/N12FINAL:2014
IMDRF/SaMD WG/N12FINAL:2014
18 sept. 2014 Authoring Group: IMDRF Software as a Medical Device (SaMD) Working Group ... produced by the International Medical Device Regulators Forum.
 Software as a Medical Device (SaMD): Key definitions
Software as a Medical Device (SaMD): Key definitions
9 déc. 2013 IMDRF/SaMD WG/N10FINAL:2013. Final Document. Title: Software as a Medical Device (SaMD): Key Definitions. Authoring Group: IMDRF SaMD ...
 IMDRF/SaMD WG/N23 FINAL: 2015 - Software as a Medical Device
IMDRF/SaMD WG/N23 FINAL: 2015 - Software as a Medical Device
2 oct. 2015 The IMDRF/SaMD WG/N12 document also highlights the use of quality management as a general consideration towards the safety effectiveness
 Final Document: Software as a Medical Device (SaMD): Clinical
Final Document: Software as a Medical Device (SaMD): Clinical
21 sept. 2017 The International Medical Device Regulators Forum (IMDRF) seeks to establish a common and converged understanding of clinical evaluation and ...
 UDI guidance: Unique Device Identification (UDI) of medical devices
UDI guidance: Unique Device Identification (UDI) of medical devices
IMDRF UDI Working Group. Date: 9 December 2013. Despina Spanou IMDRF Chair. This document was produced by the International Medical Device Regulators Forum
 IMDRF Strategic Plan 2021 - 2025
IMDRF Strategic Plan 2021 - 2025
25 sept. 2020 IMDRF International Medical. Device Regulators Forum. FINAL DOCUMENT. IMDRF Strategic Plan 2021 - 2025. IMDRF Management Committee.
 Principles and Practices for Medical Device Cybersecurity
Principles and Practices for Medical Device Cybersecurity
18 mars 2020 IMDRF. IMDRF/CYBER WG/N60FINAL:2020. International Medical. Device Regulators Forum. FINAL DOCUMENT. Principles and Practices for Medical ...
 imdrf pmd wg/n49 final: 2018
imdrf pmd wg/n49 final: 2018
18 oct. 2018 IMDRF/PMD WG/N49 FINAL:2018. Final Document. Title: Definitions for Personalized Medical Devices. Authoring Group: IMDRF Personalized ...
 MDCG 2021-10 - The status of Appendixes E-I of IMDRF N48 under
MDCG 2021-10 - The status of Appendixes E-I of IMDRF N48 under
MDCG 2021-10. Page 1 of 3. MDCG 2021-10 - The status of. Appendixes E-I of IMDRF N48 under the EU regulatory framework for medical devices. May 2021.
 IMDRF MDCE WG/N56FINAL:2019 (formerly GHTF/SG5/N2R8:2007)
IMDRF MDCE WG/N56FINAL:2019 (formerly GHTF/SG5/N2R8:2007)
IMDRF SaMD WG/N41:2017 Software as a Medical Device (SaMD): Clinical Evaluation. IMDRF Registry WG/N33FINAL:2016 Principles of International System of
