 OCCITANIE
OCCITANIE
afnor.org. OCCITANIE. Vos formations en 2019. Qualité – ISO 9001 version 2015. ISO 9001 VERSION 2015 : COMPRENDRE LES EXIGENCES. Montpellier – du 26 au 27/03 ...
 Le groupe AFNOR vient à votre rencontre dans votre région
Le groupe AFNOR vient à votre rencontre dans votre région
Pour découvrir les retours d'expérience de la mise en œuvre de l'ISO 9001 version 2015 plus de 3 ans après sa publication. ISO /IEC 17025 V2017 qualité en ...
 Rapport dactivité et de RSE du groupe AFNOR 2018
Rapport dactivité et de RSE du groupe AFNOR 2018
de management certifiés vers la version 2015 des référentiels ISO 9001 et ISO 14001 (lire page suivante). Évolution 2017 v. base. (résultats 2017). Évolution ...
 Des analyses simples et des résultats microbiologiques précis
Des analyses simples et des résultats microbiologiques précis
LA NORME ISO 9308-2:2012. CONFORMITÉ ISO 11133:2014. CERTIFICATION ISO 17025:2017. CERTIFICATION ISO 9001:2015. CERTIFICATION ISO 14001:2015. LA NORME ISO16266-
 LA VERSION ELECTRONIQUE FAIT FOI
LA VERSION ELECTRONIQUE FAIT FOI
5 fév. 2018 Au niveau français l'Afnor a publié la norme NF EN ISO/CEI 17025:2017 en remplacement de la norme NF EN. ISO/CEI 17025:2005. Ce document ...
 Calendrier des formations
Calendrier des formations
Comprendre la norme ISO 17025 V 2017 et ses exigences. À la demande. 2. Page 3 E-mail : maroc@afnor.org. Toute notre offre sur : international.afnor.com/pays ...
 presentation norme ISO 20387 b
presentation norme ISO 20387 b
Accreditation de plusieurs biobanques de tissus. ISO 17025 ; ISO 17020
 NF EN ISO/CEI 17025
NF EN ISO/CEI 17025
20 sept. 2005 ... version corrigée de 2005 de l'ISO. Afnor Normes en ligne pour: VEBIO le 20/09/2013 à 12:31. NF EN ISO/CEI 17025:2005-09. Page 4. CN 1 «Essais ...
 0045/2 - FICHES PRATIQUES QE
0045/2 - FICHES PRATIQUES QE
Ceci se traduit par la suppression des causes et par le constat de non-réapparition de la non-conformité. Nota : Les versions 2015 d'ISO 9001 et d'ISO 14001 ne
 NF EN ISO 15189
NF EN ISO 15189
14 déc. 2012 Par rapport au 1er tirage incorporation de la version corrigée de 2014 de l'ISO. Afnor
 NF EN ISO 15189
NF EN ISO 15189
14 déc. 2012 Par rapport au 1er tirage incorporation de la version corrigée de 2014 de l'ISO. Afnor
 Afnor
Afnor
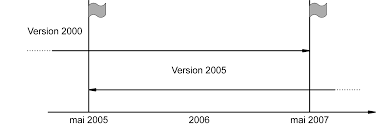
Transition de votre système qualité laboratoire à l'ISO / CEI 17025 version 2005 vers version 2017. 13 & 14 Mars ? Marseille programme
 0126/1-QE – Les exigences documentaires sont-elles allégées dans
0126/1-QE – Les exigences documentaires sont-elles allégées dans
29 juil. 2015 AFNOR Certification - Siège : 11 rue Francis de Pressensé - 93571 La Plaine ... allégées dans les versions 2015 d'ISO 9001 et d'ISO 14001?
 Sans titre
Sans titre
Anticiper les évolutions de la norme ISO/CEI 17025 www.afnor.org. Studio Groupe AFNOR • ADE • S1909120 ... 2017 Publication de la version révisée.
 presentation norme ISO 20387 b
presentation norme ISO 20387 b
Afnor juillet 2017. Approved for public release. Distribution unlimited Accreditation de plusieurs biobanques de tissus
 FICHES PRATIQUES
FICHES PRATIQUES
8 mars 2016 AFNOR Certification - Siège : 11 rue Francis de Pressensé - 93571 La ... En ISO 9001
 FICHES PRATIQUES QE – 0047/3 - Versions 2015
FICHES PRATIQUES QE – 0047/3 - Versions 2015
4 janv. 2017 AFNOR Certification - Siège : 11 rue Francis de Pressensé - 93571 La Plaine Saint-Denis ... Versions 2015. Référentiel. ISO 9001 : 2015.
 ISO/IEC 17025 - Exigences générales concernant la compétence
ISO/IEC 17025 - Exigences générales concernant la compétence
Une nouvelle version de la norme a été publiée par l'ISO et la Commission électrotechnique internationale (IEC) en 2017 afin de mettre à jour son contenu
 AFNOR NF EN ISO/CEI 17025
AFNOR NF EN ISO/CEI 17025
20 sept. 2005 Elle reproduit intégralement la Norme internationale. ISO/CEI 17025:2005 (version corrigée de 2005) et son rectificatif technique 1:2006.
 Rapport dactivité et de RSE du groupe AFNOR 2018
Rapport dactivité et de RSE du groupe AFNOR 2018
déjà identifiés par les études de 2016 et 2017 qui couvraient respectivement 9001 et ISO 14001 délivrés sur la base de la version.
 INTERNATIONAL ISO/IEC STANDARD 17025 - iasonlineorg
INTERNATIONAL ISO/IEC STANDARD 17025 - iasonlineorg
STANDARD STANDARD ISO/IEC 17025 General requirements for the competence of testing and calibration laboratories Exigences générales concernant la compétence des laboratoires d'étalonnages et d'essais Reference 17025:2017(E) ISO/IEC 2017 COPYRIGHTISO/IEC 2017 PROTECTED DOCUMENT
 ISO 17025 COMPLIANCE AND PRACTICAL GUIDELINES - OAS
ISO 17025 COMPLIANCE AND PRACTICAL GUIDELINES - OAS
ISO 17025 GENERAL REQUIREMENTS 4 Impartiality and Confidentiality •Activities Structured and Managed to Ensure Impartiality •A stated commitment from top management •Culture of Integrity What We Do •Global Quality Policy Statement •Endorsed by top management •Reviewed with each new employee •Code of Ethics
 Quality Manual-HETL FCS 17025-2017 - Maine
Quality Manual-HETL FCS 17025-2017 - Maine
ISO /IEC 17025:2017 (International Organization of Standardization / International Electrotechnical Commission (IEC) – General requirements for the competence of testing and calibration laboratories 2017 ANAB ISO/IEC GD3150 - Guiding Principles of Professional Responsibility for Forensic Service Providers and Forensic Personnel
 GUIDELINES FOR THE USE OF ACCREDITATION IN LIEU OF - NRC
GUIDELINES FOR THE USE OF ACCREDITATION IN LIEU OF - NRC
ISO/IEC 17025:2017 as the basis of the ILAC process the revision was expected to address other minor editorial changes clarifications and adjustments based on operating experience subsequent to the NRC endorsement in February 2015 The changes represented in NEI 14-05A Revision 1 are summarized as follows:
 Handbook ISO/IEC 17025:2017 - African Food Safety Network
Handbook ISO/IEC 17025:2017 - African Food Safety Network
ISO/IEC 17025:2017 ISO/IEC 17025:2005 Clause Title Clause Title 6 5 Metrological traceability 5 6 Measurement traceability Identification of changes Most of the notes have been erased and a new Informative Annex on metrological traceability has been created In Annex A possibilities have been included on how to
 Standard Guide for Developing Discipline Specific Methodology
Standard Guide for Developing Discipline Specific Methodology
Nov 12 2020 · 2 2 4 ISO/IEC 17025:2017 (E) General requirements for the competence of testing and calibration laboratories 3 Terminology 3 1 Definitions: 3 1 1 item n - object substance or material that is collected derived or sampled as part of the forensic process as defined in ISO 21043-1:2018(E)
 General Accreditation Guidance ISO/IEC 17025:2017 Gap analysis
General Accreditation Guidance ISO/IEC 17025:2017 Gap analysis
ISO/IEC 17025:2017 has adopted the revised structure specified by ISO/CASCO Accordingly the structure of the new standard is different to the 2005 version as noted below: Informative preliminary Title page Table of contents Foreword Introduction (including relationship to other standards) Normative General Title Scope Normative references
 leay:block;margin-top:24px;margin-bottom:2px; class=tit wwwipacptGUIDE FOR ISO/IEC 17025 APPLICATION - IPAC
leay:block;margin-top:24px;margin-bottom:2px; class=tit wwwipacptGUIDE FOR ISO/IEC 17025 APPLICATION - IPAC
ISO/IEC 17025 APPLICATION Page 2 of 27 OGC001 2018-12-31 1 Introduction This document provides guidance for application of ISO/IEC 17025:2017 (also referred to as ISO/IEC 17025 or as the standard) It is intended to be used by IPAC’s assessors and accredited and applicant laboratories
 CLAUSE COMPARISON CH ART ISO/IEC 17025: 2017
CLAUSE COMPARISON CH ART ISO/IEC 17025: 2017
ISO/IEC 17025: 2017 CALA has prepared a clause comparison chart to support the laboratory community’s transition to the recently published ISO/IEC 17025:2017 standard CALA is an internationally recognized accreditation body serving both public and private sector testing laboratories in Canada and abroad
 ISO / IEC 17025 2017 vs 2005 edition - EPPO
ISO / IEC 17025 2017 vs 2005 edition - EPPO
on ISO Standard 17025 (2017) and PM 7/98 (4) Laboratoryaccreditation Widely accepted process of evaluation of laboratory'squality performance reliability and efficiency Means to promote and enforce better quality inlaboratorytesting and to ultimately reduce testing errors Laboratoryaccreditation Previous Standard
 General requirements for the competence of testing and
General requirements for the competence of testing and
ISO/IEC 17025:2017(E) Introduction This document has been developed with the objective of promoting confidence in the operation of laboratories This document contains requirements for laboratories to enable them to demonstrate they operate competently and are able to generate valid results
 Searches related to iso 17025 version 2017 afnor filetype:pdf
Searches related to iso 17025 version 2017 afnor filetype:pdf
Nov 22 2021 · ISO/IEC 17025:2017 Section 7 10” Nonconforming Work” & Section 8 6 “Improvement” • This webinar is being recorded • All PJLA webinar recordings and slides are available for download from the Past Webinars section of our website –https://www pjlabs com/training/pjla-webinars • All attendees are muted However feel free to

The new ISO/IEC 17025:2017
1 | Page
The new ISO/IEC 17025:2017
By Dr. George Anastasopoulos
Director, Conformity assessment, IAS
Email: ganastasopoulos@iasonline.org
Introduction - Background information
ISO/IEC 17025
was first issued in 1999 by the International Organization for Standardization (ISO) and the International Electro-technical Commission (IEC). It is the single most important standard forcalibration and testing laboratories around the world, with more than 50.000 laboratories accredited,
globally.At the International Laboratory Accredita
tion Cooperation (ILAC) General Assembly in October 2013 the Laboratory Committee (which is composed of stakeholder representatives of accredited testing and calibration) recommended that ILAC request that ISO/CASCO establish a new work item to comprehensively revise ISO/IEC 17025:2005. CASCO is the ISO committee that works on issues relating to conformity assessment. CASCO develops policy and publishes standards related to conformity assessment; it does not perform conformity assessment activities. CASCO's standards development activities are carried out by working groups made up of experts put forward by the ISO member bodies. The experts are individuals who possess specific knowledge relating to the activities to be undertaken by the working group.The 6th
ISO/CASCO WG 44 meeting was held on July 10-12, 2017 in ISO Central Secretariat, Geneva. The deliverable of this meeting was the FDIS version of the new ISO/IEC 17025 version. The document is expected to proceed to publication, planned for end November/December 2017. Please note that throughout this article the term "the standard" refers to the new ISO/IEC 17025:2017.About the New Standard
The format of the new standard has been significantly changed to be more in line with new ISO formatting guidelines. The basic format is similar to other new standards such as ISO/IEC 17020 andISO/IEC 17065.
The new standard is now structured as follows:
1. Scope
2. Normative references
3. Terms and definitions
4. General requirements
5. Structural requirements
The new ISO/IEC 17025:2017
2 | Page
6. Resource requirements
7. Process requirements
8. Management requirements
Annex A - Metrological Traceability (Informative)
Annex B - Management System (Informative)
Bibliography
General Information
According to International Accreditation Forum (IAF) and the International Laboratory AccreditationCooperation (ILAC), accreditation is defined as
"the independent evaluation of conformity assessment bodies against recognized standards to ensure their imp artiality and competence." This standard was developed with the objective of promoting confidence in the operation of laboratories and contains requirements for laboratories to enable them to demonstrate that they operate in a competent and impartial way and that they are able to provide valid results.During its development phase it has been tried to align the standard with the principles of ISO 9001,
although this was not always practically possible. Still it is a fair statement to make that the laboratories
complying with the standard will also, in general, comply with the principles of ISO 9001. The standard can be used for accreditation purposes, for self-assessment of the laboratories and for second party assessments by laboratory customers, regulatory authorities, organizations and schemes using peer-assessment. Its requirements are applicable to any organization that performs the activities of testing and/orcalibration and/or sampling associated with subsequent testing or calibration. Therefore, accreditation
to the new standard can be also achieved by organizations offering sampling associated withsubsequent testing or calibration. When the standard uses the term "laboratory" is referring to any of
the 3 options mentioned above (testing, calibration, and sampling).The potential of performing only sampling activities is a new element in the standard. If, for example, a
laboratory is performing tests and takes samples by its own capacity, it should meet all requirements
related to both: sampling a nd testing. On the other hand, if any organization performs only sampling and then the samples are forwarded to a laboratory for testing, then this organization should comply with new standard requirements regarding sampling and its management system should ensure that the sampling activity doesn't affect negatively on test results.Requirements for sampling organizations are
similar to testing and calibration laboratories: personnel shall be competent, equipment has to be maintained and calibrated, sampling procedure has to be validated, quality of sampling has to be assured etc. Confirmation of competence of organization to provide sampling can be provided through accreditation against the new ISO/IEC 17025.Guide 99 ISO/IEC,
International vocabulary of metrology - basic and general concepts and associatedterms (VIM), is referenced in the standard as a normative reference. The definitions also given in ISO/IEC
The new ISO/IEC 17025:2017
3 | Page
17000 are applicable. In addition, the standard provides the detailed definitions of the terms
impartiality, complaint, interlaboratory comparison, intralaboratory comparison, proficiency testing,
laboratory, decision rule.Main Requirements
The Standard introduces
its main requirements throughout the clauses 4 to 8.Clause 4 - General requirements
Impartiality and Confidentiality requirements are discussed in clause 4. The risk-based thinking isevident throughout the standard. It should be noted that the new standard expects from the laboratory
to plan and implement actions to address risks and opportunities. Although addressing risks and opportunities is laboratory's responsibility, the standard sets specific requirements. The first requirement of such risks and opportunities that is needed to be addressed is mentioned in clause 4, where the labora tory is required to identify and eliminate or minimize risks related to impartiality, on an on-going basis.quotesdbs_dbs7.pdfusesText_5[PDF] iso 18091
[PDF] iso 19011
[PDF] iso 2000 definition
[PDF] iso 20000 certification
[PDF] iso 20000 definition
[PDF] iso 20000 2
[PDF] iso 20001
[PDF] iso 21500 gratuit
[PDF] iso 21500 pdf français
[PDF] iso 22000 2017
[PDF] iso 22000 définition
[PDF] iso 22000 haccp et sécurité des aliments pdf
[PDF] iso 22000 ppt presentation
[PDF] iso 22000 version 2017 pdf
