 Jasperse Acid-Base Chemistry. Extra Practice Problems
Jasperse Acid-Base Chemistry. Extra Practice Problems
A 0.100 M solution of a monoprotic weak acid has a pH of 3.00. What is the pKa of this acid? a. 5.00 d. 9.99 b. 0.999 e. 6.00.
 Acid-Base Titration and pH
Acid-Base Titration and pH
PROBLEMS Write the answer on the line to the left. Show all your work in the space provided. 4. [H3O ] in an aqueous solution 2.3 10 3 M. 4.3
 Exercise 14.3 - pH and pOH - Answers.pdf
Exercise 14.3 - pH and pOH - Answers.pdf
This value is called the ion product of water (Kw). The pH scale typically runs from 0-14 but very strong acids may have negative pH's
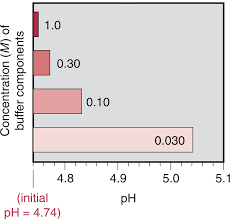 Section 19.1. Acid-Base Buffer Solutions
Section 19.1. Acid-Base Buffer Solutions
solution. (B) Calculate new pH (or pOH …) after something is added. Example: Sample Problem 19.1. (1) Calculate pH of a solution that is 0.50 M HAc and 0.50 M ...
 Exercise 15.4 - Titrations - Answers.pdf
Exercise 15.4 - Titrations - Answers.pdf
Solving Titration Problems. A titration is a chemical process for finding the The pH of any strong acid Istrong base titration at the equivalence point.
 Solutions to Review Problems for Acid/Base Chemistry
Solutions to Review Problems for Acid/Base Chemistry
1) are further diluted to exactly 800. mL the solution pH is 2.74. Calculate Ka for acetic acid. First
 1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
Explain how a buffer solution manages to stabilize the pH against the addition of acid base
 1 Graphical Solution of Acid/Base Problems Graphical Solution of
1 Graphical Solution of Acid/Base Problems Graphical Solution of
• Stronger bases become protonated at higher pH than weaker bases. All available exchangeable. H+ ions are used to protonate available bases
 [PDF] Test2 ch17a Acid-Base Practice Problems
[PDF] Test2 ch17a Acid-Base Practice Problems
p3 Recognizing Acid/Base Properties when Ionics are Dissolved in Water p11 pH Calculations; Relationships between pH and pOH p4 Answers
 [PDF] Solutions to Review Problems for Acid/Base Chemistry
[PDF] Solutions to Review Problems for Acid/Base Chemistry
1) are further diluted to exactly 800 mL the solution pH is 2 74 Calculate Ka for acetic acid First there is a dilution followed by an equilibrium
 [PDF] Calculating pH and pOH worksheet Everett Community College
[PDF] Calculating pH and pOH worksheet Everett Community College
What is the pH of a 0 0235 M HCl solution? 2) What is the pOH of a Since there is both acid and base we will assume a 1 mole acid:1 mole base ratio of
 [PDF] Chapter 11 – Acids and Bases – Practice Problems Section 111
[PDF] Chapter 11 – Acids and Bases – Practice Problems Section 111
An Arrhenius acid produces H+ and an Arrhenius base produces OH- in aqueous solutions If you know the [OH-] how can you determine the pH of a solution?
 [PDF] Acid and Base pH Calculations – Supplemental Worksheet KEY
[PDF] Acid and Base pH Calculations – Supplemental Worksheet KEY
We will calculate the pH of the solutions using the following 3 steps for each problem Step 1: What is left in solution? Step 2: What are the equilibrium
 [PDF] Calculate the pH of the solution that results when 400 mL
[PDF] Calculate the pH of the solution that results when 400 mL
Worksheet: Acid base problems - AP level Problems 1 - 10 Problem #1: Calculate the pH of the solution that results when 40 0 mL of 0 100 M
 [PDF] Keys Acid Base Ch 15 pdf
[PDF] Keys Acid Base Ch 15 pdf
A solution of acid has [H] = 3 0 x 10³ M pH = 5 $47 pH = 8 597 7 A solution of base has an [OH] = 4 25 x 10 M Solve the problems below
 [PDF] 19_A_B_C_Worksheet_Keypdf - Humble ISD
[PDF] 19_A_B_C_Worksheet_Keypdf - Humble ISD
An acidic solution has a pH 7 0 BASE d pOH = 1 4 12 6 BASE 6 Classify the solutions in Problem 5 as acidic or basic
 [PDF] ACID – BASE EQUILIBRIUM
[PDF] ACID – BASE EQUILIBRIUM
3- Calculate the pH of the solution of all sorts of acids bases and A typical strong acid problem might be: What is the pH of a C a M HCl solution?
 [PDF] Acid-Base Titration and pH - David Brearley High School
[PDF] Acid-Base Titration and pH - David Brearley High School
The [H3O ] is 4 6 10 8 M in a solution Calculate the [OH ] PROBLEMS Write the answer on the line to the left Show all your work in the space provided
[PDF] acid base reaction problems with answers
[PDF] acid base titration problems with answers
[PDF] acid base titration problems with answers pdf
[PDF] acid base test review answers
[PDF] acid/base stoichiometry practice problems answers
[PDF] acide acétique
[PDF] acide base ph cours
[PDF] acide base ph exercice
[PDF] acide base ph terminale s
[PDF] acide base physique chimie
[PDF] acide base physique terminale s
[PDF] acide base physique ts
[PDF] acide et base conjuguée
[PDF] acide et base de bronsted
