 Exercise 15.4 - Titrations - Answers.pdf
Exercise 15.4 - Titrations - Answers.pdf
Solving Titration Problems. A titration is a chemical process for finding the The pH of any strong acid Istrong base titration at the equivalence point.
 ACID-BASE TITRATIONS (PROBLEMS)
ACID-BASE TITRATIONS (PROBLEMS)
being titrated with 0.50 M NaOH. Calculate the pH of this solution initially before any NaOH is added. At this point
 Problem Solving
Problem Solving
Apr 20 2016 Mole ratio of base to acid in titration reaction ? Volume of base solution titrated. 20.00 mL. Moles of base in solution titrated ? mol NaOH.
 Acid-Base Titration and pH
Acid-Base Titration and pH
PROBLEMS Write the answer on the line to the left. Show all your work in the space provided. 4. [H3O ] in an aqueous solution 2.3 10 3 M. 4.3
 Acid – Base Titration Calculations (WA + SB)
Acid – Base Titration Calculations (WA + SB)
Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid HC3H5O3 after it has been titrated with a total of 30.0 ml of 0.40 M KOH? Page 2. P G4 A (pg of ).
 1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
What Kind of Solution/pH at End? p2 Titration Calculations p11. Preparation and Recognition of Buffers p4 pH Estimations/Calculations after acid/base are
 Untitled
Untitled
EXTRA PRACTICE: Titration Problems Practice. Titration Calculate the moles of base used the moles of acid used and the concentration of the original acid.
 Acid – Base Titration Calculations (WB + SA)
Acid – Base Titration Calculations (WB + SA)
1. Calculate the pH of 20.0 ml of a 0.060 M solution of ammonia NH3. 2. What volume of 0.040 M HI would be needed to
 Test3 ch17b Buffer-Titration-Equilibrium Practice Problems
Test3 ch17b Buffer-Titration-Equilibrium Practice Problems
Answer: A buffer consists of a weak acid and its conjugate base in roughly equal amounts. If acid is added to the solution it is consumed by the conjugate
 What is the M of NaOH if it takes 40.0 ml of NaOH to reach the
What is the M of NaOH if it takes 40.0 ml of NaOH to reach the
Worksheet - Titration Problems. 1. What is the M of NaOH if it takes 40.0 ml H₂Soy as Hel to neutralize the base. 30.3 ml of 0.305 M NaOH are required to ...
 w336-titrations-worksheet.pdf
w336-titrations-worksheet.pdf
calculations for the concentration of the base? 3). It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuric acid solution
 Exercise 15.4 - Titrations - Answers.pdf
Exercise 15.4 - Titrations - Answers.pdf
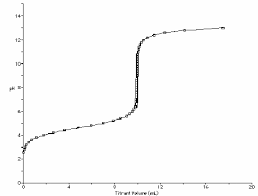
Solving Titration Problems While the volumes of acid and base should probably be converted to liters as long as they are the same unit
 Acid – Base Titration Calculations (WA + SB)
Acid – Base Titration Calculations (WA + SB)
Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid HC3H5O3 after it has been titrated with a total of 30.0 ml of 0.40 M KOH? Page 2. P G4 A (pg of ).
 Titration Calculations Strong Acid/Strong Base Calculations (1) Use
Titration Calculations Strong Acid/Strong Base Calculations (1) Use
added during a titration to 25.0 mL of a 0.12M HCl solution with 0.15M NaOH solution? For strong acid/base titration perform stoichiometry calculation
 Bookmark File PDF Acid Base Titration Lab 39 Answers ? - covid19
Bookmark File PDF Acid Base Titration Lab 39 Answers ? - covid19
easy to acquire as without difficulty as download lead Acid Base Titration. Lab 39 Answers. It will not say yes many grow old as we accustom before.
 1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
What Kind of Solution/pH at End? p2 Titration Calculations p11. Preparation and Recognition of Buffers p4 pH Estimations/Calculations after acid/base.
 Where To Download Redox Practice Problems With Answers Copy
Where To Download Redox Practice Problems With Answers Copy
with states phases
 A Dating Analogy for Acid-Base Titration Problems
A Dating Analogy for Acid-Base Titration Problems
alleviate this confusion. The use of other analogies to help clarify other confusing concepts related to solution concen- trations and stoichiometric ratios
 Worksheet 22 – Weak Acid/Strong Base Titrations A. Initial pH This
Worksheet 22 – Weak Acid/Strong Base Titrations A. Initial pH This
This can only be used after the initial point and before the equivalence point when the acid and its conjugate base are both major species in the solution. At
 Test3 ch17b Buffer-Titration-Equilibrium Practice Problems
Test3 ch17b Buffer-Titration-Equilibrium Practice Problems
What Kind of Solution/pH at End? p2 Titration Calculations p11. Preparation and Recognition of Buffers p4 pH Estimations/Calculations after acid/base.
[PDF] acid base test review answers
[PDF] acid/base stoichiometry practice problems answers
[PDF] acide acétique
[PDF] acide base ph cours
[PDF] acide base ph exercice
[PDF] acide base ph terminale s
[PDF] acide base physique chimie
[PDF] acide base physique terminale s
[PDF] acide base physique ts
[PDF] acide et base conjuguée
[PDF] acide et base de bronsted
[PDF] acide et base de lewis
[PDF] acide et base exercices corrigés pdf
[PDF] acide et base pdf
