 Exercise 15.4 - Titrations - Answers.pdf
Exercise 15.4 - Titrations - Answers.pdf
Solving Titration Problems. A titration is a chemical process for finding the The pH of any strong acid Istrong base titration at the equivalence point.
 ACID-BASE TITRATIONS (PROBLEMS)
ACID-BASE TITRATIONS (PROBLEMS)
being titrated with 0.50 M NaOH. Calculate the pH of this solution initially before any NaOH is added. At this point
 Problem Solving
Problem Solving
Apr 20 2016 Mole ratio of base to acid in titration reaction ? Volume of base solution titrated. 20.00 mL. Moles of base in solution titrated ? mol NaOH.
 Acid-Base Titration and pH
Acid-Base Titration and pH
PROBLEMS Write the answer on the line to the left. Show all your work in the space provided. 4. [H3O ] in an aqueous solution 2.3 10 3 M. 4.3
 Acid – Base Titration Calculations (WA + SB)
Acid – Base Titration Calculations (WA + SB)
Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid HC3H5O3 after it has been titrated with a total of 30.0 ml of 0.40 M KOH? Page 2. P G4 A (pg of ).
 1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
What Kind of Solution/pH at End? p2 Titration Calculations p11. Preparation and Recognition of Buffers p4 pH Estimations/Calculations after acid/base are
 Untitled
Untitled
EXTRA PRACTICE: Titration Problems Practice. Titration Calculate the moles of base used the moles of acid used and the concentration of the original acid.
 Acid – Base Titration Calculations (WB + SA)
Acid – Base Titration Calculations (WB + SA)
1. Calculate the pH of 20.0 ml of a 0.060 M solution of ammonia NH3. 2. What volume of 0.040 M HI would be needed to
 Test3 ch17b Buffer-Titration-Equilibrium Practice Problems
Test3 ch17b Buffer-Titration-Equilibrium Practice Problems
Answer: A buffer consists of a weak acid and its conjugate base in roughly equal amounts. If acid is added to the solution it is consumed by the conjugate
 What is the M of NaOH if it takes 40.0 ml of NaOH to reach the
What is the M of NaOH if it takes 40.0 ml of NaOH to reach the
Worksheet - Titration Problems. 1. What is the M of NaOH if it takes 40.0 ml H₂Soy as Hel to neutralize the base. 30.3 ml of 0.305 M NaOH are required to ...
 w336-titrations-worksheet.pdf
w336-titrations-worksheet.pdf
calculations for the concentration of the base? 3). It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuric acid solution
 Exercise 15.4 - Titrations - Answers.pdf
Exercise 15.4 - Titrations - Answers.pdf
Solving Titration Problems While the volumes of acid and base should probably be converted to liters as long as they are the same unit
 Acid – Base Titration Calculations (WA + SB)
Acid – Base Titration Calculations (WA + SB)
Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid HC3H5O3 after it has been titrated with a total of 30.0 ml of 0.40 M KOH? Page 2. P G4 A (pg of ).
 Titration Calculations Strong Acid/Strong Base Calculations (1) Use
Titration Calculations Strong Acid/Strong Base Calculations (1) Use
added during a titration to 25.0 mL of a 0.12M HCl solution with 0.15M NaOH solution? For strong acid/base titration perform stoichiometry calculation
 Bookmark File PDF Acid Base Titration Lab 39 Answers ? - covid19
Bookmark File PDF Acid Base Titration Lab 39 Answers ? - covid19
easy to acquire as without difficulty as download lead Acid Base Titration. Lab 39 Answers. It will not say yes many grow old as we accustom before.
 1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
What Kind of Solution/pH at End? p2 Titration Calculations p11. Preparation and Recognition of Buffers p4 pH Estimations/Calculations after acid/base.
 Where To Download Redox Practice Problems With Answers Copy
Where To Download Redox Practice Problems With Answers Copy
with states phases
 A Dating Analogy for Acid-Base Titration Problems
A Dating Analogy for Acid-Base Titration Problems
alleviate this confusion. The use of other analogies to help clarify other confusing concepts related to solution concen- trations and stoichiometric ratios
 Worksheet 22 – Weak Acid/Strong Base Titrations A. Initial pH This
Worksheet 22 – Weak Acid/Strong Base Titrations A. Initial pH This
This can only be used after the initial point and before the equivalence point when the acid and its conjugate base are both major species in the solution. At
 Test3 ch17b Buffer-Titration-Equilibrium Practice Problems
Test3 ch17b Buffer-Titration-Equilibrium Practice Problems
What Kind of Solution/pH at End? p2 Titration Calculations p11. Preparation and Recognition of Buffers p4 pH Estimations/Calculations after acid/base.
P G4 A (pg ! of !) Acid - Base Titration Calculations (WA + SB) Name___________________Per____14Consider the changes (pH, [H+
], [OH-] ) when titrating 50.0 ml of 0.20 M solution of lactic acid, HC3H5O3 with 0.40 M potassium hydroxide. Then construct a titration curve on the graph on page 2. The Ka of lactic acid = 1.4 × 10-4
1.Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid, HC3H5O3. 2.What volume of 0.40 M KOH would be needed to neutralize this 50.0 ml of a 0.20 M solution of lactic acid, HC3H5O3? (This should be the first step you would take to solve any titration problem, even if you weren't asked to do it.) 3.Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid, HC3H5O3 after it has been titrated with 10.0 ml of 0.40 M KOH? 4.Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid, HC3H5O3 after it has been titrated with a total of 12.5 ml of 0.40 M KOH? 5.Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid, HC3H5O3 after it has been titrated with a total of 20.0 ml of 0.40 M KOH? 6.Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid, HC3H5O3 after it has been titrated with a total of 25.0 ml of 0.40 M KOH? 7.Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid, HC3H5O3 after it has been titrated with a total of 30.0 ml of 0.40 M KOH?
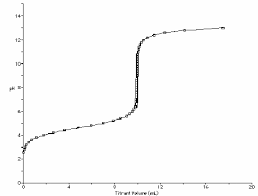
P G4 A (pg ! of !) Acid - Base Titration Calculations (WA + SB) Name___________________Per____24Make a sketch of the titration curve using the volumes and pH values calculated for the titration
of 50 ml of 0.20 M lactic acid with 0.40 M KOH. Placing a point on the graph for problems 3 - 7 from the previous page and #'s 8 & 9 below. As you construct this graph, you will get a much more accurate sense of the curve if you make two more calculations and place two more points on the graph. 8.Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid, HC3H5O3 after it has been titrated with a total of 24.0 ml of 0.40 M KOH? 9.Calculate the pH of 50.0 ml of a 0.20 M solution of lactic acid, HC3H5O3 after it has been titrated with a total of 26.0 ml of 0.40 M KOH?
pH#Volume#of#KOH#added#(ml)#Titra5on#curve#for#WA#+#SB#
P G4 A (pg ! of !) Acid - Base Titration Calculations (WA + SB) ANSWERS34Be sure that if you need help, you refer to NS G.1 - Acid-Base Titrations. First you need to recognize that this is a WA being titrated with a SB. 1.This is an "x2
" problem. x = [H+ ] = 5.3 10-3M so pH = 2.28 2.It is ALWAYS important to calculate the volume required to reach the equivalence point; moles of acid = moles of base, MaVa = MbVb •so 0.20 M × 50 ml = 0.40 M × Vb •thus the volume of strong base needed to reach the equivalence point is 25.0 ml of KOH 3.At this point you are only partway through the titration, but not yet to the halfway point. •Calculate the millimoles of lactic acid (0.2M × 50ml) and millimoles of incoming strong base (0.4M × 10ml) •Subtract the incoming strong base from the weak acid, to find out the remaining un-neutralized weak acid (6 mmole) and there so very little A-
in solution to start but the incoming strong base will drive the equilibrium to the right and produce an amount of mmoles of A-
equivalent to the incoming strong base (4 mmole) so you will add that to the conjugate weak base (which was originally ~ 0) !! •Solve and [H+
] = 2.1 10-4M so pH = 3.68 4.Yippee! This is the halfway point of the titration, because half of the weak acid is reacted (10 mmol - 5 mmol), and half of the conjugate weak base forms (5 mmol) ! and thus [HA] = [A-] resulting in !! •so always at the halfway point, the Ka = [H+
] = 1.4 10-4and pH = 3.85 5.At this point you are again only partway through the titration. •Calculate the millimoles of lactic acid (0.2 M × 50 ml) and millimoles of incoming strong base (0.4M × 20ml) •Subtract the incoming strong base from the weak acid, to find out the remaining un-neutralized acid (2 mmole) and there is so very little A-
in solution to start, ~0, but the incoming base will drive the equilibrium to the right and produce an amount of mmoles of A-
equivalent to the incoming strong base (8 mmole) so you will add that to the conjugate weak base (which was originally ~ 0) !! •Solve [H
] = 3.5× 10
5M so pH = 4.46
6.At this point you are at the equivalence point and all of the WA has been neutralized - the equilibrium has been driven all the way to the right, but the pH is NOT 7 because there will be conjugate WB, (A-
) in the solution which does not just "sit there," but hydrolyzes with water causing the pH to be above 7. So here you must switch gears. •You have already calculated the millimoles of WA, and that will be the amount of conjugate WB, A-
in solution because the incoming strong base has driven the neutralization "all the way" and all the WA has been converted to WB. •thus [A-
] = 10 mmole divided by total volume (75 ml) = 0.133 M •You can then use the [A- ] in a simple "x2 " WB calculation to determine the x = [OH- ] • (Of course you need to calculate the Kb from the Ka remember: Ka × Kb = 1 × 10-14 •thus and x = [OH- ] = 3.1 10-6M •After solving for x = [OH-
] you can log[OH-] determine the pOH = 5.51 the "14-it" for pH = 8.49 7.At this point in the titration the amount of strong base added exceeds the amount of weak acid. •You know that the equivalence point occurs at 25 ml of KOH added, thus 30 ml of KOH is 5 ml beyond the equivalence point resulting in an extra 2 mmole of strong base (since 0.40 M × 5 ml extra base = 2 mmol extra SB) (OR 10mm WA with 12 mmol SB = 2 mm extra SB) •Divide this extra 2 mmole of strong base by total volume (80 ml) to get 0.025 M of strong base in excess. (The rest of the incoming strong base was neutralized by H+
put out by the dissociating WA.) This SB which contributes far more OH- than the conjugate weak base does, so you need not concern yourself with any minute "extra" OH- contributed by the WB. •Thus -log[0.025] = pOH = 1.60, thus "14-it" to get pH = 12.40P G4 A (pg ! of !) Acid - Base Titration Calculations (WA + SB) ANSWERS448.At this point, just like the calculation that you made in #5, you are again only partway through the titration. •Calculate the millimoles of lactic acid (0.2 M × 50 ml) and millimoles of incoming strong base (0.4M × 24ml) •Subtract the incoming strong base from the weak acid, to find out the remaining un-neutralized weak acid (0.4 mmole). Realize that there so very little A-
in solution to start but the incoming strong base will drive the equilibrium to the right and produce an amount of mmoles of A-
equivalent to the incoming SB (9.6 mmole) so you will add that to the conjugate WB (which was originally ~ 0) !! •Solve [H
] = 5.8× 10
6M so pH = 5.23
9.At this point in the titration the amount of SB added exceeds the amount of WA, just like in #7. •You know that the equivalence point occurs at 25 ml of KOH added, thus 26 ml of KOH is 1 ml beyond the equivalence point. From previous calculations, we know that the mmole of WA is (0.2 M × 50 ml) 10 mmoles, and the mmol of incoming SB is more, 10.4 mmoles (0.40 M × 26 ml SB) •Subtract to get 0.4 mmole of excess SB, then divide by total volume (76 ml) to get 0.0055 M of strong base in excess. (The rest of the incoming SB was neutralized by H+
put out by the dissociating WA.) This SB which contributes far more OH-than the conjugate weak base contributes, so you need not concern yourself with any minute "extra" OH-
pH#Volume#of#KOH#added#(ml)#Titra5on#curve#for#WA#+#SB#
quotesdbs_dbs10.pdfusesText_16[PDF] acid base test review answers
[PDF] acid/base stoichiometry practice problems answers
[PDF] acide acétique
[PDF] acide base ph cours
[PDF] acide base ph exercice
[PDF] acide base ph terminale s
[PDF] acide base physique chimie
[PDF] acide base physique terminale s
[PDF] acide base physique ts
[PDF] acide et base conjuguée
[PDF] acide et base de bronsted
[PDF] acide et base de lewis
[PDF] acide et base exercices corrigés pdf
[PDF] acide et base pdf
