 8-Synthesis-of-Aspirin.pdf
8-Synthesis-of-Aspirin.pdf
If acetic anhydride is used instead of acetic acid the reaction is much faster and has a higher yield (since acetic anhydride is much more reactive than acetic
 An Efficient Microscale Procedure for the Synthesis of Aspirin
An Efficient Microscale Procedure for the Synthesis of Aspirin
Jun 6 1998 The recommended procedures for the synthesis of aspirin use either acetic anhydride–concentrated sulfuric acid (1) or phosphoric acid (2) as the ...
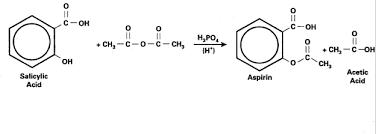 The Synthesis of a Medicinal Agent- Aspirin By Walter Scharf and
The Synthesis of a Medicinal Agent- Aspirin By Walter Scharf and
*The chemical equation corresponding to the synthesis reaction is: C7H6O3 +. (CH3CO)2O. →. C9H804 +. CH3COOH. Salicylic acid Acetic anhydride. Aspirin. Acetic
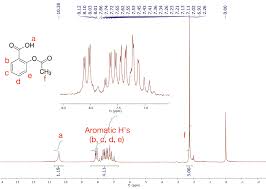 Yin and Yang in Chemistry Education: The Complementary Nature
Yin and Yang in Chemistry Education: The Complementary Nature
Aspirin was synthesized from the reaction of salicylic acid and acetic anhydride whereas wintergreen oil was made via the reaction of salicylic acid and
 Preparation of Solid Acid–Activated Carbon as Catalyst in Aspirin
Preparation of Solid Acid–Activated Carbon as Catalyst in Aspirin
opposite result was observed in the acetic anhydride application in the synthesis. The logical reason behind this result was still unknown It might be
 Pharmaceutical Chemistry Synthesis of Aspirin Principle : Synthesis
Pharmaceutical Chemistry Synthesis of Aspirin Principle : Synthesis
Salicylic acid interacts with acetic anhydrides in presence of few drops of conc. sulphuric acid to produce aspirin and a molecule of acetic acid. The
 Core practical 16: Synthesise aspirin from 2-hydroxybenzoic acid
Core practical 16: Synthesise aspirin from 2-hydroxybenzoic acid
Add 5.0 cm3 of ethanoic anhydride to the. 2-hydroxybenzoic acid. Add five drops of concentrated sulfuric acid to the mixture in the flask. Fix a condenser on
 Yin and yang in chemistry education: the complementary nature of
Yin and yang in chemistry education: the complementary nature of
Figure 5: Typical chemical shift ranges in 1H NMR spectroscopy. Figure 6: IR spectra acquired from aspirin synthesis. Page 4. In the acetic anhydride reactant
 5012 Synthesis of acetylsalicylic acid (aspirin) from salicylic acid
5012 Synthesis of acetylsalicylic acid (aspirin) from salicylic acid
A mixture of 13.8 g (100 mmol) salicylic acid and. 12.8 g (11.9 mL 125 mmol) acetic anhydride is filled in the reaction flask and three drops of conc.
 Experiment 8 – Synthesis of Aspirin
Experiment 8 – Synthesis of Aspirin
Aspirin can be made by reacting salicylic acid with acetic acid in the presence If acetic anhydride is used instead of acetic acid the reaction is much ...
 Experiment 5 - Synthesis of Aspirin
Experiment 5 - Synthesis of Aspirin
The synthesis reaction of aspirin is shown below: Since acetic acid is very soluble in water it is easily separated from the aspirin product. The aspirin.
 ACETIC ANHYDRIDE HAZARD SUMMARY IDENTIFICATION
ACETIC ANHYDRIDE HAZARD SUMMARY IDENTIFICATION
perfumes explosives and aspirin. REASON FOR CITATION. * Acetic Anhydride is on the Hazardous Substance List because it is regulated by OSHA and cited by
 The Synthesis of a Medicinal Agent- Aspirin By Walter Scharf and
The Synthesis of a Medicinal Agent- Aspirin By Walter Scharf and
A convenient way to obtain complete reaction in the preparation of an acetate ester is to use acetic anhydride. The reaction is catalyzed by concentrated
 Aspirin synthesis from salicylic acid.
Aspirin synthesis from salicylic acid.
Company is synthesized by the conversion of Salicylic acid (SA) to Acetylsalicylic acid (ASA) using acetic anhydride in the presence of phosphoric acid
 SYNTHESIS OF ASPIRIN
SYNTHESIS OF ASPIRIN
In this experiment aspirin will be produced by reacting salicylic acid with acetic anhydride
 Untitled
Untitled
set-up the aspirin synthesis reaction continue with synthesis of aspirin ... Ethanoic anhydride. 2-Ethanoyloxybenzene carboxylic acid. (aspirin).
 E29 Preparation of Aspirin (Acetylsalicylic Acid) and Thin-Layer
E29 Preparation of Aspirin (Acetylsalicylic Acid) and Thin-Layer
Experiment 1: Synthesis of acetylsalicylic acid (aspirin). Acetic anhydride and salicylic acid react to produce acetylsalicylic acid and acetic acid;
 ASPIRIN SYNTHESIS
ASPIRIN SYNTHESIS
We just make two acetic acid molecules to react with each other and they form oxide link between. 6. Why do we use acetic anhydride instead of acetic acid?
 Preparation of Solid Acid–Activated Carbon as Catalyst in Aspirin
Preparation of Solid Acid–Activated Carbon as Catalyst in Aspirin
with acetic anhydride in the acetylation reaction produced an overall yield as high Keywords: Solid Acid Catalyst Activated Carbon

Aspirin synthesis from salicylic acid.
Introduction
The Introduction is written in Present Tense. Brief history and technique overview. Acetylsalicylic acid (ASA), which is being sold under the trade name "aspirin" by the Bayer Company is synthesized by the conversion of Salicylic acid (SA) to Acetylsalicylic acid (ASA) using acetic anhydride in the presence of phosphoric acid acting as a catalyst. The reaction involves the conversion of a phenol to an ester. This reaction is reversible, which is why aspirin commonly smells of vinegar, as the ASA hydrolyzes in air back to SA and acetic acid.Reaction :
Iron (III) chloride (FeCl3) reacts with phenols to form brightly colored complexes, thus providing an excellent means for testing the effectiveness of the ASA to SA conversion. (ASA does not react because the phenol group is acetylated.Experimental Procedure:
Past Tense. What did you do exactly? Someone of similar training should be able to repeat the experiment using these instructions and obtain your results.2.0 grams of salicylic acid, 5.0 mL of acetic anhydride and 5 drops of 85% phosphoric acid
solution were placed into a 50 mL Erlenmeyer flask. A 70-80 °C hot water bath was prepared by placing a 250 mL beaker on a hot plate with a thermometer to monitor temperature. The 50 mL Erlenmeyer flask with the mixture of salicylic acid, acetic anhydride, and phosphoric acid was partially submerged in the water bath and heated for 15 minutes until vapors ceased to be released. After 10 minutes of heating the submerged flask passed, 2 mL of distilled water was added to the flask. Then, once the reaction reached completion the flask was removed and 20 mL of distilled water was added. The flask was left to cool to room temperature before being placed in an ice bath for 5 minutes to allow crystallization to occur. A vacuum filtration was set up and the mixture was filtered via vacuum filtration. Once the liquid had been drawn out of the mixture, the crystals were washed with 5 mL of cold, distilled water. This was repeated once more. The vacuum filtration apparatus was left on for several minutes to aid in the drying of the solid product before it was weighed and recorded. Crude acetylsalicylic acid (aspirin) was added to a 125 mL Erlenmeyer flask. About 60 mL of hot ethanol/water solvent was added slowly to the crude aspirin in a warm water bath. Once the crystals dissolved, the flask was covered and left to cool to room temperature before it was placed in an ice bath for 10 minutes to fully crystallize. Then, the crystals were placed into a vacuum filter where they were subsequently rinsed with two 3 mL portions of cold deionized water and one 2 mL portion of cold ethanolResults and Discussion:
(What did you discover?) Your observations of the experiment as it progresses is important, new information. Write these observations (color changes, appearance of crystals, formation of an emulsion, boiling temperatures,test results, etc.) in your notebook as you do the experiment. Also record the weights of reagents and
products and tare weights in this section. Specific sources of error must be noted Figure 1: Structure of Salicylic Acid, Acetic anhydride, and Acetyl salicylic acid The structures of salicylic acid, acetic anhydride, and acetylsalicylic acid are pictured above with their functional groups clearly visible in red.Table 1: Synthesis of Aspirin Data
Mass of salicylic acid used (g) 2.009 g
Volume of acetic anhydride used (mL) 5.000 mL
Mass of acetic anhydride used (1.08 g/mL)
used (g)5.400 g
Mass of aspirin and filter paper (g) 3.159 g
Mass of filter paper (g) 0.130 g
Mass of crude aspirin synthesized (g) 3.029 g
Mass of purified aspirin product (g) 2.169 g
The table above depicts the various masses and volumes of calculated and raw data in the synthesis of aspirin. 2.009 grams of salicylic acid was used with 5.000 mL of acetic anhydride. nown density for a mass of5.400 grams. The mass of aspirin and filter paper was 3.159 grams. The mass of the filter paper
was .1300 grams. Thus, the calculated value of crude synthesized aspirin was 3.029 grams. Following purification, the calculated mass of the final aspirin product was 2.169 grams.Theoretical Yield
2.0 g salicylic acid (1 mole/138.0 g) = 0.014 moles
5 mL acetic anhydride (1.08 g/mL) = 5.4 g
5.4 g (1 mole/102 g) = 0.05 moles
There is a smaller molar amount of salicylic acid so it is the limiting reagent. Therefore, the theoretical yield of acetylsalicylic acid is 0.014 moles.0.014 moles acetylsalicylic acid (180 g/mole) = 2.52 g
Percent Yield = (experimental mass/theoretical mass) x 100%Percent Yield = (2.169/2.520) x 100
Percent Yield = 86.07 %
Table 2: Theoretical Yield, Percent Error, and Percent YieldTheoretical Yield (g) 2.520 g
Percent Yield 86.02 %
The calculated theoretical yield was 2.520 grams. Thus, the percent error was 13.93 % and the percent yield was 86.07%. The esterification reaction is a term for a general reaction in which two reactants, an alcohol and an acid, form an ester in the final product. This reaction can be used to synthesize aspirin from salicylic acid. These types of reactions are typically reversible, so most esterification reactions are equilibrium imposed on a system that is in equilibrium will cause the system to adjust to a new equilibrium in order to counteract the change. The reaction is slow in pure acetic anhydride, therefore phosphoric acid was used as a catalyst amount of acetic anhydride would force the equilibrium towards the desired product, acetylsalicylic acid. This mechanism would cause the reaction to favor the product side (aspirin and acetic acid).The solution was also heated in order to accelerate the approach to equilibriumIt is important to consider that acetylsalicylic acid is not the only product that forms, acetic acid
is another byproduct of the reaction. The objective is to isolate pure acetylsalicylic acid. A hot water/ ethanol mixture (about 20 mL hot solvent of water/ethanol per gram crude aspirin) is used to further purify aspirin by removing acetic acid. The acetic acid is very soluble in water and can be removed from aspirin, which is less polar and interacts with the ethanol portion of the mixture. A purified product is obtained after recrystallization of crude aspirin in the hot ethanol.Conclusion:
A total of 2.169 grams of pure aspirin was synthesize out of a possible yield of 2.52 grams. Thus, there was 86.07% product yield. Acetylation of salicylic acid makes aspirin less acidic and therefore less damaging to the digestive system of the human body. In the future, special care should be given to the washing of the crystals with cold distilled water to maximize yield. Also, a stronger acid catalyst such sulfuric acid could be used to further increase the rate of reaction.quotesdbs_dbs2.pdfusesText_4[PDF] acetic anhydride ir spectrum
[PDF] acetic anhydride is obtained by the reaction of acetic acid and
[PDF] acetic anhydride ka formula
[PDF] acetic anhydride lewis structure
[PDF] acetic anhydride line formula
[PDF] acetic anhydride literature boiling point
[PDF] acetic anhydride literature melting point
[PDF] acetic anhydride melting boiling point
[PDF] acetic anhydride molecular formula
[PDF] acetic anhydride molecular melting point
[PDF] acetic anhydride molecular weight
[PDF] acetic anhydride msds fisher
[PDF] acetic anhydride msds fisher scientific
[PDF] acetic anhydride msds merck
