 8-Synthesis-of-Aspirin.pdf
8-Synthesis-of-Aspirin.pdf
If acetic anhydride is used instead of acetic acid the reaction is much faster and has a higher yield (since acetic anhydride is much more reactive than acetic
 An Efficient Microscale Procedure for the Synthesis of Aspirin
An Efficient Microscale Procedure for the Synthesis of Aspirin
Jun 6 1998 The recommended procedures for the synthesis of aspirin use either acetic anhydride–concentrated sulfuric acid (1) or phosphoric acid (2) as the ...
 The Synthesis of a Medicinal Agent- Aspirin By Walter Scharf and
The Synthesis of a Medicinal Agent- Aspirin By Walter Scharf and
*The chemical equation corresponding to the synthesis reaction is: C7H6O3 +. (CH3CO)2O. →. C9H804 +. CH3COOH. Salicylic acid Acetic anhydride. Aspirin. Acetic
 Yin and Yang in Chemistry Education: The Complementary Nature
Yin and Yang in Chemistry Education: The Complementary Nature
Aspirin was synthesized from the reaction of salicylic acid and acetic anhydride whereas wintergreen oil was made via the reaction of salicylic acid and
 Preparation of Solid Acid–Activated Carbon as Catalyst in Aspirin
Preparation of Solid Acid–Activated Carbon as Catalyst in Aspirin
opposite result was observed in the acetic anhydride application in the synthesis. The logical reason behind this result was still unknown It might be
 Pharmaceutical Chemistry Synthesis of Aspirin Principle : Synthesis
Pharmaceutical Chemistry Synthesis of Aspirin Principle : Synthesis
Salicylic acid interacts with acetic anhydrides in presence of few drops of conc. sulphuric acid to produce aspirin and a molecule of acetic acid. The
 Core practical 16: Synthesise aspirin from 2-hydroxybenzoic acid
Core practical 16: Synthesise aspirin from 2-hydroxybenzoic acid
Add 5.0 cm3 of ethanoic anhydride to the. 2-hydroxybenzoic acid. Add five drops of concentrated sulfuric acid to the mixture in the flask. Fix a condenser on
 Yin and yang in chemistry education: the complementary nature of
Yin and yang in chemistry education: the complementary nature of
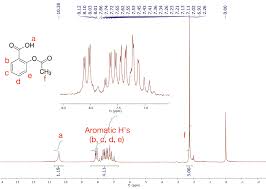
Figure 5: Typical chemical shift ranges in 1H NMR spectroscopy. Figure 6: IR spectra acquired from aspirin synthesis. Page 4. In the acetic anhydride reactant
 Aspirin synthesis from salicylic acid.
Aspirin synthesis from salicylic acid.
This reaction is reversible which is why aspirin commonly smells of vinegar
 5012 Synthesis of acetylsalicylic acid (aspirin) from salicylic acid
5012 Synthesis of acetylsalicylic acid (aspirin) from salicylic acid
A mixture of 13.8 g (100 mmol) salicylic acid and. 12.8 g (11.9 mL 125 mmol) acetic anhydride is filled in the reaction flask and three drops of conc.
 Experiment 8 – Synthesis of Aspirin
Experiment 8 – Synthesis of Aspirin
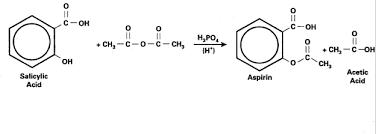
Aspirin can be made by reacting salicylic acid with acetic acid in the presence If acetic anhydride is used instead of acetic acid the reaction is much ...
 Experiment 5 - Synthesis of Aspirin
Experiment 5 - Synthesis of Aspirin
The synthesis reaction of aspirin is shown below: Since acetic acid is very soluble in water it is easily separated from the aspirin product. The aspirin.
 ACETIC ANHYDRIDE HAZARD SUMMARY IDENTIFICATION
ACETIC ANHYDRIDE HAZARD SUMMARY IDENTIFICATION
perfumes explosives and aspirin. REASON FOR CITATION. * Acetic Anhydride is on the Hazardous Substance List because it is regulated by OSHA and cited by
 The Synthesis of a Medicinal Agent- Aspirin By Walter Scharf and
The Synthesis of a Medicinal Agent- Aspirin By Walter Scharf and
A convenient way to obtain complete reaction in the preparation of an acetate ester is to use acetic anhydride. The reaction is catalyzed by concentrated
 Aspirin synthesis from salicylic acid.
Aspirin synthesis from salicylic acid.
Company is synthesized by the conversion of Salicylic acid (SA) to Acetylsalicylic acid (ASA) using acetic anhydride in the presence of phosphoric acid
 SYNTHESIS OF ASPIRIN
SYNTHESIS OF ASPIRIN
In this experiment aspirin will be produced by reacting salicylic acid with acetic anhydride
 Untitled
Untitled
set-up the aspirin synthesis reaction continue with synthesis of aspirin ... Ethanoic anhydride. 2-Ethanoyloxybenzene carboxylic acid. (aspirin).
 E29 Preparation of Aspirin (Acetylsalicylic Acid) and Thin-Layer
E29 Preparation of Aspirin (Acetylsalicylic Acid) and Thin-Layer
Experiment 1: Synthesis of acetylsalicylic acid (aspirin). Acetic anhydride and salicylic acid react to produce acetylsalicylic acid and acetic acid;
 ASPIRIN SYNTHESIS
ASPIRIN SYNTHESIS
We just make two acetic acid molecules to react with each other and they form oxide link between. 6. Why do we use acetic anhydride instead of acetic acid?
 Preparation of Solid Acid–Activated Carbon as Catalyst in Aspirin
Preparation of Solid Acid–Activated Carbon as Catalyst in Aspirin
with acetic anhydride in the acetylation reaction produced an overall yield as high Keywords: Solid Acid Catalyst Activated Carbon

SYNTHESIS OF ASPIRIN
I. OBJECTIVES AND BACKGROUND
You will:
synthesize acetylsalicylic acid (aspirin) by carrying out a simple organic reaction, separate your product from the reaction mixture by vacuum filtration, purify your product by recrystallization, perform a chemical test to identify the change in functional group from reactant to product, and determine the success of your synthesis by calculating the percentage yield of your product.INTRODUCTION
Aspirin is one of the most widely used
medications in the world. It is employed as an analgesic (pain relief), an anti-pyretic (fever control) and an anti-inflammatory. More recently, studies have indicated that daily intake of small doses of aspirin can lower the risk of heart attack and stroke in high-risk patients. The history of aspirin and its precursor dates back to ancient times. Documents attributed to Hippocrates, the father of modern medicine, from the 4th century B.C. refer to the alleviation of pain by chewing on the bark of a willow tree or ingesting a powder made from the bark and leaves of the willow. This remedy was passed on from generation to generation. Fast forward now to the 19th century, where the field of organic chemistry began to experience tremendous growth. By 1838, chemists had managed to isolate, purify and identify the component of willow bark that provided the analgesic benefit. The compound was named salicylic acid, which was based on the genus name of the willow. Efforts to market salicylic acid met with failure, due to an unfortunate side effect-- prolonged ingestion of salicylic acid led to stomach pain, and in some cases, ulcers. Inspection of the structure of salicylic acid molecule (see figure below) sheds some light on the cause of this problem. Salicylic acid contains two functional groups, a carboxylic acid (-COOH) and an alcohol (-OH). The carboxylic acid group, as the name implies, has a tendency to generate H3O+ in aqueous solution. In addition, since the alcohol group is bound to a benzene ring, it belongs to a special class of alcohols known as phenols. Phenols have many unique properties, one of which being that they are substantially more acidic than other types of alcohols. As a result, the two acidic functional groups serve to lower the pH of the stomach, leading to the gastric distress described above. Figure 29-1. Structures of aspirin and salicylic acid Chemists sought to modify the salicylic acid molecule, reasoning that modification of one of the functional groups could lower the acidity of the compound without affecting the medical benefits. This was achieved by taking advantage of some fundamental organic chemistry. When an alcohol is allowed to react with a carboxylic acid in the presence of an acid catalyst, the functional groups combine in a condensation reaction to form an ester and water: By reacting the phenol group of salicylic acid with acetic acid, an ester group (which is not acidic) is formed and the resulting compound was named acetylsalicylic acid. Although this reaction was first achieved in the 1850's, it wasn't until 1899 that scientists at the Bayer company in Germany identified acetylsalicylic acid as a commercially viable compound. They gave it the brand name aspirin (derived from the arcane genus name of meadowsweet, another natural source of salicylic acid). The drug was in high demand almost immediately, and Bayer was soon on its way to becoming a pharmaceutical giant.Synthesis
In this experiment, aspirin will be produced by reacting salicylic acid with acetic anhydride, a derivative of acetic acid that has similar chemicals properties to acetic acid, but reacts more rapidly and efficiently. to form acetylsalicylic acid (aspirin). Note in Equation (29-3) that the byproduct of the reaction is now acetic acid rather than water.Recrystallization
A chemical reaction seldom occurs in such a manner that the desired product is obtained in an absolutely pure state. The synthetic reaction in this experiment will be no exception. The acetic anhydride will be added in excess, so unreacted acetic anhydride will be present at the end of the reaction. In addition, sulfuric acid (added as a catalyst) and acetic acid product will also contaminate the product. Failure to remove these acidic impurites would defeat the purpose of the reaction, which is to lower the acidity of the product compared to the salicylic acid starting material! Consequently, a purification procedure must be carried out to separate the aspirin from such impurities as unused starting materials and the acetic acid by-product. Recrystallization is an extremely useful technique for purifying crystalline solids. The impure product is dissolved in a minimum volume of hot solvent. The solvent is carefully selected so that the product is much less soluble in cold solvent than hot, so that the product will recrystallize when the solvent is cooled, leaving the soluble impurities behind in the solvent. The pure product is then isolated by vacuum filtration.Iron (III) Chloride Test for Phenols
A convenient test can be carried out to identify the change in functional group from reactant to product. A comparison of the structural formulas of aspirin and salicylic acid shows that the salicylic acid has a phenolic group while aspirin has none. A specific test for a phenolic group will illustrate this change in functionality. If a drop of iron (Ill) chloride (FeCl3) solution is added to an aqueous solution containing traces of a phenolic group, a color change occurs. The color varies from reds to blues to greens depending on the particular phenol molecule present.quotesdbs_dbs2.pdfusesText_4[PDF] acetic anhydride ir spectrum
[PDF] acetic anhydride is obtained by the reaction of acetic acid and
[PDF] acetic anhydride ka formula
[PDF] acetic anhydride lewis structure
[PDF] acetic anhydride line formula
[PDF] acetic anhydride literature boiling point
[PDF] acetic anhydride literature melting point
[PDF] acetic anhydride melting boiling point
[PDF] acetic anhydride molecular formula
[PDF] acetic anhydride molecular melting point
[PDF] acetic anhydride molecular weight
[PDF] acetic anhydride msds fisher
[PDF] acetic anhydride msds fisher scientific
[PDF] acetic anhydride msds merck
