 Core practical 4: Investigate the hydrolysis of halogenoalkanes
Core practical 4: Investigate the hydrolysis of halogenoalkanes
Pour 5 cm3 of silver nitrate solution into three clean test tubes. Now place the test tubes in the water bath. 5. When the halogenoalkane–ethanol solutions have
 Copy of Practical Flashcards - CP5 Rate of Hydrolysis of
Copy of Practical Flashcards - CP5 Rate of Hydrolysis of
What is the name of the mechanism for the hydrolysis of halogenoalkanes? Describe an experiment to investigate the hydrolysis of halogenoalkanes. 1. Fill ...
 Practical notes - CP5 Investigating the Rates of Hydrolysis of
Practical notes - CP5 Investigating the Rates of Hydrolysis of
Investigating the Rates of Hydrolysis of. Halogenoalkanes www.pmt.education. Page 2. Method. 1. Set up 3 test tubes each with 1 cm3 of ethanol and two drops
 Halogenoalkanes - Edexcel Chemistry A-level
Halogenoalkanes - Edexcel Chemistry A-level
*(ii) Devise an experiment to compare the rates of hydrolysis of 2-chlorobutane. 2-bromobutane and 2-iodobutane. State the trend in the rates of reaction.
 Chemistry
Chemistry
Jun 12 2018 6 This is a question about the hydrolysis of halogenoalkanes. (a) Devise an experiment
 1. The kinetics of the hydrolysis of the halogenoalkane RCH2Cl with
1. The kinetics of the hydrolysis of the halogenoalkane RCH2Cl with
(iii) In an experiment designed to find the mechanism of the reaction between 2-bromo-. 2-methylpropane and hydroxide ions the following data were obtained at
 CP 04 - Rate of hydrolysis of halogenoalkanes
CP 04 - Rate of hydrolysis of halogenoalkanes
Rates of hydrolysis of halogenoalkanes. www.pmt.education. Page 2. Method. 1. Set up 3 test tubes each with 1 cm3 of ethanol and two drops of a haloalkane
 Chemistry - Unit AS2: Practical Manual
Chemistry - Unit AS2: Practical Manual
Halogenoalkanes undergo hydrolysis forming alcohols when they react with water. Give two assumptions made about the reaction mixture in this experiment. 3 ...
 Halogenoalkanes and Alcohols
Halogenoalkanes and Alcohols
Identification of halogenoalkanes – Reaction with Silver nitrate solution. In the presence of water halogenoalkanes undergo hydrolysis. The halogenoalkanes
 Core practical 4: Investigate the hydrolysis of halogenoalkanes
Core practical 4: Investigate the hydrolysis of halogenoalkanes
Pour 5 cm3 of silver nitrate solution into three clean test tubes. Now place the test tubes in the water bath. 5. When the halogenoalkane–ethanol solutions have
 Practical notes - CP5 Investigating the Rates of Hydrolysis of
Practical notes - CP5 Investigating the Rates of Hydrolysis of
Repeat steps 3 and 4 for the remaining 2 haloalkanes. Key points. ? This is a ?nucleophilic substitution?reaction where water acts as the nucleophile. (
 Copy of Practical Flashcards - CP5 Rate of Hydrolysis of
Copy of Practical Flashcards - CP5 Rate of Hydrolysis of
Describe an experiment to investigate the hydrolysis of halogenoalkanes. 1. Fill three test tubes with 5 cm3 of ethanol and add 4 drops of a haloalkane to.
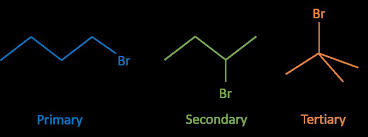
 Classifying Halogenoalkanes Reactions of Halogenoalkanes
Classifying Halogenoalkanes Reactions of Halogenoalkanes
Hydrolysis is defined as the splitting of a molecule ( in this If the experiment is repeated using primary secondary and tertiary halogenoalkanes
 Chemistry
Chemistry
12 juin 2018 6 This is a question about the hydrolysis of halogenoalkanes. (a) Devise an experiment giving outline details only
 CP 04 - Rate of hydrolysis of halogenoalkanes
CP 04 - Rate of hydrolysis of halogenoalkanes
Method. 1. Set up 3 test tubes each with 1 cm?3? of ethanol and two drops of a haloalkane. [iodo/bromo/chloro alkanes]. 2. Place the test tubes in a water
 Investigating the rate of hydrolysis of organic halogen compounds
Investigating the rate of hydrolysis of organic halogen compounds
This is an example of a hydrolysis reaction. Thorpe A. Experimental error and error analysis: just how good are those.
 chemrevise
chemrevise
Comparing the rate of hydrolysis reactions. Water is a poor nucleophile but it can react slowly with haloalkanes in a substitution reaction.
 1. The kinetics of the hydrolysis of the halogenoalkane RCH2Cl with
1. The kinetics of the hydrolysis of the halogenoalkane RCH2Cl with
(iii) In an experiment designed to find the mechanism of the reaction between 2-bromo-. 2-methylpropane and hydroxide ions the following data were obtained at
 Subject: Chemistry Topic: Halogenoalkanes 3.3.3 Year Group: 12
Subject: Chemistry Topic: Halogenoalkanes 3.3.3 Year Group: 12
7 Hydrolysis. The breaking of chemical bonds with water. 8 Elimination. Reaction. A reaction in which a small molecule is removed from the organic compound.
 Core practical 4: Investigate the hydrolysis of halogenoalkanes
Core practical 4: Investigate the hydrolysis of halogenoalkanes
The hydrolysis of halogenoalkanes is a nucleophilic substitution reaction In this investigation the nucleophile is water If NaOH is used to hydrolyse the halogenoalkanes then any excess NaOH has to be neutralised by HNO3 before adding AgNO3 Questions Write an equation for the reaction of 1-bromobutane with water
 Hydrolysis of Halogenoalkanes: Conditions StudySmarter
Hydrolysis of Halogenoalkanes: Conditions StudySmarter
Measure and record the time taken for the precipitates to form (this is a measure ofthe rate of reaction) Repeat steps 3 and 4 for the remaining 2 haloalkanes Key points This is a nucleophilic substitution reaction where water acts as the nucleophile (hydrolysis) Precipitation with Ag+: RX + H X? (aq)
 Investigating the rate of hydrolysis of organic halogen compounds
Investigating the rate of hydrolysis of organic halogen compounds
Experiment Starter Sheet – Investigating the hydrolysis of organic halogen compounds Here is a suggested method to investigate the rate of the reaction Prepare the following solutions • 2 - bromo 2 -methylpropane • Ethanol/water mixture 4:1 vol/vol • Sodium hydroxide 0 05 mol dm-3 • Methyl red indicator
 33 Halogenoalkanes - chemrevise
33 Halogenoalkanes - chemrevise
Feb 3 2021 · Comparing the rate of hydrolysis reactions Water is a poor nucleophile but it can react slowly with halogenoalkanes in a substitution reaction Hydrolysis is defined as the splitting of a molecule ( in this case a halogenoalkane) by a reaction with water CH3CH2X + H2O CH3CH2OH + X-+ H+ Aqueous silver nitrate is added to a halogenoalkane The
 33 Halogenoalkanes - chemrevise
33 Halogenoalkanes - chemrevise
Feb 3 2018 · 2 Elimination reaction of halogenoalkanes Elimination: removal of small molecule (often water) from the organic molecule Elimination with alcoholic hydroxide ions Change in functional group: halogenoalkane alkene Reagents: Potassium (or sodium) hydroxide Conditions: In ethanol; Heat Mechanism: Elimination Type of reagent: Base OH-C C H H H Br
 Searches related to hydrolysis of halogenoalkanes experiment filetype:pdf
Searches related to hydrolysis of halogenoalkanes experiment filetype:pdf
a) The relative rates of hydrolysis of the following halogenoalkanes can be determined experimentally: Chloropropane Bromobutane Iodobutane I Put the above halogenoalkanes in order with the most reactive first II Explain their relative reactivity III Describe a simple chemical experiment to show how you would determine their
What is hydrolysis of halogenoalkanes?
- Hydrolysis is decomposition of a chemical due to reaction with water. hydrolysis of halogenoalkanes is a nucleophilic substitution type of reaction.
What happens if NaOH is used to hydrolyse halogenoalkanes?
- The hydrolysis of halogenoalkanes is a nucleophilic substitution reaction. In this investigation the nucleophile is water. If NaOH is used to hydrolyse the halogenoalkanes, then any excess NaOH has to be neutralised by HNO3 before adding AgNO3. Write an equation for the reaction of 1-bromobutane with water. In these reactions a precipitate forms.
Which aqueous solution is used to make a halogen iodoalkane reaction?
- An aqueous solution of sodium hydroxide (NaOH) or potassium hydroxide (KOH) with ethanol is used This reaction is very slow at room temperature, so the reaction mixture is warmed The rate of this reaction depends on the type of halogen in the halogenoalkane Fluoroalkanes do not react at all, but iodoalkanes have a very fast rate of reaction
How do you compare the rate of reaction between haloalkanes and hydrolysis?
- The rate of reaction can be compared by carrying out the reaction in the presence of silver ions. The halogen atoms in haloalkanes are covalently bonded to carbon and give no precipitate of a silver halide. Hydrolysis releases halide ions, which immediately precipitate as the silver halide.
[PDF] hydrolysis of lactose mechanism
[PDF] hydrolysis of maleic anhydride mechanism
[PDF] hydrolysis of maleic anhydride to maleic acid mechanism
[PDF] hydrolysis of methyl acetate graph
[PDF] hydrolysis of nucleic acid
[PDF] hydrolysis of nucleic acid yields
[PDF] hydrolysis of paracetamol
[PDF] hydrolysis of polyesters
[PDF] hydrolysis of polysaccharides experiment
[PDF] hydrolysis of polysaccharides proteins and lipids experiment
[PDF] hydrolysis of polysaccharides with trifluoroacetic acid
[PDF] hydrolysis of starch benedict's test
[PDF] hydrolysis of starch by acid
[PDF] hydrolysis of starch by acid lab report
