 Qualitative analysis of Carbohydrates II
Qualitative analysis of Carbohydrates II
Experiment 1 : Sucrose Hydrolysis Test. Objective: This test is used to convert sucrose (non-reducing disaccharide) to glucose + fructose (reducing mono.
 Experiment 11 – Carbohydrates
Experiment 11 – Carbohydrates
In this experiment you will hydrolyze a sample of sucrose and then test it for the presence of a reducing sugar. You will also hydrolyze a sample of starch and
 Chem 11 Lab Manual
Chem 11 Lab Manual
The iodine test is used in this experiment to indicate the completion of the hydrolysis. Hydrolysis of Sucrose (Acid versus Base Catalysis). Sample. Condition ...
 THE HYDROLYSIS OF SUCROSE BY HYDROCHLORIC ACID IN
THE HYDROLYSIS OF SUCROSE BY HYDROCHLORIC ACID IN
was frequently determined during the course of an experiment. The flask containing the solution remaining after filling the polarimeter tubes was immersed in a
 Inverted sugar syrup attained from sucrose hydrolysis using a
Inverted sugar syrup attained from sucrose hydrolysis using a
In all tests the pH (5.5)
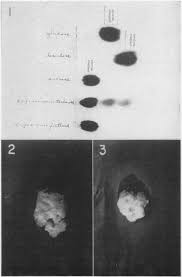 Hydrolysis of Sucrose by Autoclaving Media a Neglected Aspect in
Hydrolysis of Sucrose by Autoclaving Media a Neglected Aspect in
They made no chemical tests or chromatograms of the sugars in the autoclaved sucrose medium. Callus cultures of Sequoia grown on these two differently
 TESTSFOR CARBOHYDRATES FATSAND PROTEINS
TESTSFOR CARBOHYDRATES FATSAND PROTEINS
(iii) Polysaccharides : These yield large number of monosaccharide units on hydrolysis. Disaccharides do not give this test. Test for Sucrose. Hydrolyse the ...
 The Enzymatic Hydrolysis of Sucrose
The Enzymatic Hydrolysis of Sucrose
The enzyme invertase (sucrase saccharase) catalyzes the hydrolysis of sucrose (a disaccharide) to invert sugar. Glucose and fructose both test positively ...
 Sucrose Hydrolysis in a Continuous Packed-Bed Reactor with Auto
Sucrose Hydrolysis in a Continuous Packed-Bed Reactor with Auto
١٣/٠٧/٢٠٢١ Sucrose (0.1 M) in 0.1 M acetate buffer at pH 5 was used as substrate for enzymatic activity assays. Test tubes with 0.9 mL of the substrate and ...
 Hydrolysis of High Concentration Sucrose Solution into Glucose and
Hydrolysis of High Concentration Sucrose Solution into Glucose and
2.4 Stability and reusability test. The Amberlyst-15 catalyst was pretreated before the reusability test using a vacuum filter to separate the catalyst from
 Qualitative analysis of Carbohydrates II
Qualitative analysis of Carbohydrates II
Experiment 1 : Sucrose Hydrolysis Test. Objective: This test is used to convert sucrose (non-reducing disaccharide) to glucose + fructose (reducing mono.
 The Enzymatic Hydrolysis of Sucrose
The Enzymatic Hydrolysis of Sucrose
Benedict's qualitative solution is a test reagent that reacts positively with (simple) reducing sugars. All monosaccharides and most disaccharides are reducing
 Chem 11 Lab Manual
Chem 11 Lab Manual
Hydrolyzed sucrose (a mixture of D–glucose and D–fructose) will give a positive test with Benedict's or Fehling's reagent as well as hydrolyzed amylose (a
 measured individually. The rate of hydrolysis of sucrose in vivo has
measured individually. The rate of hydrolysis of sucrose in vivo has
gastro-intestinal tract. Absorption tests. The fasting rats were given either 760 mg sucrose (on hydrolysis this amount will yield 800 mg invert sugar)
 Experiment 11 – Carbohydrates
Experiment 11 – Carbohydrates
Starch pectin
 EXPERIMENT 1- QUALITATIVE ANALYSIS OF CARBOHYDRATES
EXPERIMENT 1- QUALITATIVE ANALYSIS OF CARBOHYDRATES
Perform this test with fructose glucose
 Production of invertase enzymes from Saccharomyces cerevisiae
Production of invertase enzymes from Saccharomyces cerevisiae
To test the sucrose hydrolysis activity ofimmobilized and non-immobilized form of Saccharomyces cerevisiae in sucrose broth and quantitative and
 Activities and the Hydrolysis of Sucrose with Concentrated Acids
Activities and the Hydrolysis of Sucrose with Concentrated Acids
Concentrated Acid Hydrolysis of Sucrose. [Contribution from hydrolysis with equivalent concentration of hy- ... when the experiment was to be started.
 Inverted sugar syrup attained from sucrose hydrolysis using a
Inverted sugar syrup attained from sucrose hydrolysis using a
sucrose hydrolysis using a cell-type membrane reactor (CTMR) coupled with an ultra (UF-100kDa)
 Effect of pressure on the hydrolysis of sucrose by invertase
Effect of pressure on the hydrolysis of sucrose by invertase
pressure on the rate of hydrolysis of sucrose by immobilized invertase. the substrate reservoir at 5 min intervals and placed in a test tube immersed.
 Experiment 15 Carbohydrates - Moorpark College
Experiment 15 Carbohydrates - Moorpark College
Hydrolysis of Sucrose (Acid versus Base) 1 Place 3 mL of 2 sucrose solution in each of two labeled test tubes To the first test tube (#1) add 3 mL of water and 3 drops of dilute sulfuric acid solution (3 M H 2 SO 4 To the second test tube (#2) add 3 mL of water and 3 drops of dilute sodium hydroxide solution (3 M NaOH)
 (PDF) Contribution on Determination of the Rate Constant
(PDF) Contribution on Determination of the Rate Constant
Jan 11 2012 · Hydrolysis of Sucrose 7 Add 0 5 mL of 3 M HCl to 5 mL of a 1 sucrose solution in a test tube Mix Heat and stir the mixture in a boiling water bath for 20 minutes (You may add deionized water to this solution if the volume starts getting low!) Cool the solution and add 1 M NaOH until the solution tests neutral on litmus paper (To
 SUCROSE D-FRUCTOSE and D-GLUCOSE - Megazyme
SUCROSE D-FRUCTOSE and D-GLUCOSE - Megazyme
Hydrolysis of sucrose: At pH 4 6 sucrose is hydrolysed by ?-fructosidase to D-glucose and D-fructose (?-fructosidase) (5) Sucrose + H2O D-glucose + D-fructose The D-glucose in the sample following hydrolysis of sucrose (total D-glucose) is determined as described above
 External Hydrolysis of Sucrose Application Note
External Hydrolysis of Sucrose Application Note
AND SUCROSE UTILIZING EXTERNAL HYDROLYSIS Application Note YSI Life Sciences • 204LS-02 Introduction Dextrose (D-glucose) and sucrose concentrations in complex matrices can be measured directly and quickly using either a YSI 2500 Glucose/Lactate Analyzer or a YSI 2900 Series Biochemistry Analyzer YSI’s unique enzyme
 Searches related to hydrolysis test for sucrose filetype:pdf
Searches related to hydrolysis test for sucrose filetype:pdf
Test-Combination for 22 assays each references (A 2 B 2 C 2 D 2) Principle (Ref A 1) The D-glucose concentration is determined before and after the enzymatic hydrolysis of sucrose; D-fructose is determined subsequently to the determination of D-glucose Determination of D-glucose before inversion:
How to study the hydrolysis of sucrose?
- For studying the hydrolysis of sucrose there is different methods. The angle of rotation can be determined by polarimetry. Then we can obtain the constant of rate of reaction, k. In this study, the hydrolysis of sucrose 1/20 M was investigated by using HCl as catalyst and in presence and absence of methanol in 20, 24 and 28oC.
How do you test for sucrose and D-glucose?
- Determination of sucrose, D-glucose and D-fructose in fermentation samples and cell culture media Place the sample (after centrifugation, if necessary) in a waterbath at 80°C for 15 min to stop enzymatic reactions. Centrifuge and use the supernatant (diluted according to the dilution table, if necessary) for the assay.
What is the sucrose hemolysis test?
- SUCROSE HEMOLYSIS TEST The sucrose hemolysis test is used as a confirmatory test for paroxysmal nocturnal hemoglobinuria (PNH) when the sugar water test is positive.
Does hydrolyzed sucrose give a positive test with Benedict's reagent?
- Hydrolyzed sucrose (a mixture of D–glucose and D–fructose) will give a positive test with Benedict’s or Fehling’s reagent as well as hydrolyzed amylose (a mixture of glucose and glucose–containing oligosaccharides).
66 Experiment 11 - Carbohydrates Carbohydrates are a class of natural compounds that contain either an aldehyde or a ketone group and many hydroxyl groups - they are often called polyhydroxy aldehydes or ketones. A monosaccharide consists of a single carbohydrate molecule, containing between 3 and 7 carbons. Examples of monosaccharides are glucose and fructose. A disaccharide consists of two monosaccharides that are linked together. Sucrose and lactose are disaccharides. A polysaccharide consists of many monosaccharides linked together. Starch, pectin, glycogen, and cellulose are examples of polysaccharides. Carbohydrates are used for energy. The carbohydrates that we eat are broken down in our bodies and eventually form water and carbon dioxide. The energy obtained in this process is used for other reactions that must occur in the body. Excess carbohydrates that we eat can be stored in the liver as glycogen or can be converted to fats. Plants create carbohydrates in the process of photosynthesis, where energy from the sun is used to build carbohydrates from water and carbon dioxide. Monosaccharide structures can be written as Fischer projections. In a Fischer projection, the structure is drawn vertically with the carbonyl carbon at the top. It is understood that for each chiral carbon in the molecule, the horizontal bonds point out of the page (toward you) and the vertical bonds point into the page (away from you). Fischer projections are used to indicate the stereochemistry of each chiral carbon in the molecule and to compare monosaccharide structures easily. For example, there are many aldohexoses with the same connections of atoms but different stereochemistry, and they all have different names! The Fischer projections for glucose and galactose are shown below. Note that the only difference between these sugars is the stereochemistry around carbon 4, yet they have different names. In solution, most monosaccharides exist in a cyclic form - the aldehyde or ketone group reacts with one of the -OH groups on the other end of the same molecule to form a cyclic hemiacetal. Shown here are the cyclic structures for D-glucose. Notice that there are two possibilities: α-D-glucose and β-D-glucose. These are called the different anomers of glucose. In solution, there is an equilibrium between the cyclic form and the open chain or free aldehyde form. The rings are constantly opening up and closing again. In this way, the alpha and beta forms can be interconverted. C
OH OHH HHO OHH OHH CH 2 OHFischer projection
for D-glucose C OH OHH HHO HHO OHH CH 2 OHFischer projection
for D-galactose OCyclic form for
α-D-glucose
CH 2 OH OH OH OH OH OCyclic form for
β-D-glucose
CH 2 OH OH OH OH OH67 Chemical Tests for Carbohydrates A reducing sugar is one that can be oxidized. In order to be a reducing sugar, the molecule must contain a free anomeric carbon, since it is the open-chain form of the aldehyde that is able to react (and be oxidized). One test for reducing sugars involves Fehling's reagent, which contains Cu2+ ions in an aqueous basic solution. If a reducing agent is present, the Cu2+ is reduced to Cu+ and forms a red precipitate of Cu2O. Therefore, if Fehling's solution is added to a solution containing a reducing sugar, a red precipitate will form. Sometimes the reaction mixture must be heated in order for the precipitate to form. The color of the precipitate can vary from red to orange to green (the green color is actually a mixture of an orange and a blue precipitate). Barfoed's test is similar to Fehling's test, except that in Barfoed's test, different types of sugars react at different rates. Barfoed's reagent is much milder than Fehling's reagent. Reducing monosaccharides react quickly with Barfoed's reagent, but reducing disaccharides react very slowly or not at all. Therefore, it is possible to distinguish between a reducing monosaccharide and a reducing disaccharide using Barfoed's reagent. A positive test is a dark red precipitate and is evidence of a reducing monosaccharide. In Seliwanoff's test, a dehydration reaction is involved. Seliwanoff's reagent contains a non-oxidizing acid (HCl) and resorcinol. When a ketose is reacted with this reagent, it becomes dehydrated and a cherry-red complex forms (not a precipitate). Aldoses also react with this reagent, but much more slowly than ketoses. When Seliwanoff's reagent is reacted with a disaccharide or a polysaccharide, the acid in the solution will first hydrolyze them into monosaccharides, and the resulting monosaccharides can then be dehydrated. Disaccharides and polysaccharides will therefore react slowly with Seliwanoff's reagent. When you carry out this test, it is important to note the time required for a reaction to occur. Iodine forms a blue, black, or gray complex with starch and is used as an experimental test for the presence of starch. The color of the complex formed depends on the structure of the polysaccharide and the strength and age of the iodine solution. Iodine does not form a complex with simpler carbohydrates (monosaccharides and disaccharides). Amylose (starch) is helically coiled in solution, and it is this helical structure that is necessary to form the blue complex with iodine. Monosaccharides and disaccharides are too small to be helically coiled. Amylopectin, cellulose, and glycogen form different colors with iodine - red, brown, or purple. Many carbohydrates can undergo fermentation in the presence of yeast. The carbohydrate is the food source for the yeast, and the products of the fermentation reaction are ethanol and carbon dioxide gas. C6H12O6 à 2 CH3CH2OH + 2 CO2 (g) Glucose Ethanol Fermentation is used in the processes of making beer and wine, where the alcohol produced by the yeast is the desired product. Not all sugars, however, can be used by yeast as a food source. You will test which sugars ferment in the presence of yeast and which ones do not. The evidence of fermentation will be the evolution of carbon dioxide gas. In the test, a quantity of a solution (containing yeast and the sugar to be tested) will be trapped in an upside-down small test tube. After a few days, you will check to see if a gas bubble has formed in the test tube. If there is a gas bubble, it means fermentation did occur. Disaccharides and polysaccharides can be hydrolyzed in the presence of acid or specific enzymes. When a disaccharide is hydrolyzed, the products are the individual
68 monosaccharides. When a polysaccharide is hydrolyzed, the products will depend on how long the mixture is allowed to react, the concentration of acid or enzyme, and other factors. Polysaccharides are very long and have many glycosidic bonds to hydrolyze. They cannot all be hydrolyzed at the same time, so the product is a mixture of dextrins, maltose, and glucose. If a polysaccharide sample is hydrolyzed completely (which means that it must react for a while), the product is glucose. In this experiment, you will hydrolyze a sample of sucrose and then test it for the presence of a reducing sugar. You will also hydrolyze a sample of starch and then test it for the presence of both a reducing sugar and starch. Safety Precautions: • Wear your safety goggles. Waste Disposal: • All waste must be placed in the inorganic waste containers (which have a blue label) in one of the fume hoods. Procedure Fehling's Test 1. In this part of the experiment, you will test glucose, fructose, lactose, sucrose, starch, and your unknown. Add 6 drops of the solution to be tested to each of 6 labeled test tubes. In a larger test tube, mix 6 mL of Fehling's solution A with 6 mL of Fehling's solution B. Add 2 mL of this mixture to each of the 6 test tubes, and mix each tube thoroughly by shaking the tube well. Place these tubes in a boiling water bath for 5 minutes. After 5 minutes, remove the tubes from the water bath and record your observations. The formation of a red precipitate indicates a positive reaction. Barfoed's Test 2. You will again test glucose, fructose, lactose, sucrose, starch, and your unknown. Add 1 mL of the solution to be tested to each of 6 labeled test tubes. Add 3 mL of Barfoed's reagent to each of the 6 test tubes, and mix each tube thoroughly by shaking the tube. Place these tubes in a boiling water bath for 5 minutes. After 5 minutes, remove the tubes from the water bath, let them cool, and then cool them further by running cold water over the outside of each test tube. Record your observations. The formation of a red precipitate indicates a positive reaction. Note the amount of time needed for the red precipitate to occur in each case. Seliwanoff's Test 3. For this part, you will test glucose, fructose, lactose, water, and your unknown. Add 10 drops of the solution to be tested to each of 5 labeled test tubes. Add 4 mL of Seliwanoff's reagent to each of the 5 test tubes, and mix each tube thoroughly by shaking the tube. Place these tubes in a boiling water bath and note the time needed for any color change to occur. After 10 minutes, stop heating the tubes. Record your observations. A color change indicates a positive reaction. Iodine Test
69 4. You will test glucose, fructose, lactose, sucrose, starch, water, and your unknown. Add 1 mL of the solution to be tested to each of 7 labeled test tubes. Add 3 drops of iodine solution to each of the 7 test tubes, and mix each tube. Compare the colors and record your observations. Fermentation Test 5. In this part of the experiment, you will test glucose, fructose, lactose, sucrose, starch, water, and your unknown. Obtain 7 large test tubes, label them, and fill each one with the solution to be tested. Add of yeast to each of the tubes and mix well with a stirring rod to dissolve the yeast. (Make sure to rinse off the stirring rod between solutions so as not to contaminate them.) Put a small test tube upside-down in each large test tube. Cover the top of each large test tube with parafilm or a rubber stopper and invert the large tube so that the small test tube inside gets filled with the solution. Return the large test tube to an upright position, and remove the rubber stopper or parafilm. The small test tube inside should now be completely filled with solution - it should not have any air bubbles in it. (If it does, try again!) When each tube has been prepared, set them aside in your laboratory drawer until the next laboratory period. 6. At the next laboratory period, check to see if there is a gas bubble in any of the small test tubes. The presence of a gas bubble is evidence that a gas was produced in the reaction. If a gas was produced, that means that fermentation occurred in the tube. Record your observations. Hydrolysis of Sucrose 7. Add 0.5 mL of HCl to 5 mL of a 1 % sucrose solution in a test tube. Mix. Heat and stir the mixture in a boiling water bath for 20 minutes. (You may add deionized water to this solution if the volume starts getting low!) Cool the solution, and add NaOH until the solution tests neutral on litmus paper. (To test the solution, you will need both red and blue litmus paper. Add a drop of NaOH to the solution, stir it with a stirring rod, and then touch the stirring rod to each piece of litmus paper. Repeat this until neither piece of litmus paper changes color. A basic solution will turn the red litmus paper blue, and an acidic solution will turn blue litmus red. If the solution is neutral, neither piece of litmus paper will change color.) 8. Transfer 8-10 drops of this solution to a small test tube. In a separate tube, mix together 1 mL of Fehling's solution A with 1 mL of Fehling's solution B. Add this mixture to the small test tube containing your hydrolyzed sucrose, and heat for a few minutes in a boiling water bath. Record your observations. Compare the results of this test with your results for unhydrolyzed sucrose in step 1 of this experiment. Hydrolysis of Starch 9. Place 3 mL of 1 % starch in a test tube and add 0.5 mL of HCl. Mix and place this mixture in a boiling water bath for 10 minutes. After 10 minutes, remove the tube from the water bath and let it cool. Neutralize this solution with 1 M NaOH and mix well (use the same procedure for neutralization that you used in step 7 above). 10. Transfer 8-10 drops of this solution to a small test tube. (Save the rest of it for step 11.) In a separate tube, mix together 1 mL of Fehling's solution A with 1 mL of Fehling's solution B. Add this mixture to the small test tube containing your hydrolyzed starch, and heat for a few minutes in a boiling water bath. Record your observations. Compare the results of this test with your results for unhydrolyzed starch in step 1 of this experiment.
70 11. Using your solution from the end of step 9 (the hydrolyzed starch solution), transfer 1 mL to a small test tube. Add 3 drops of the iodine solution, and record your observations. Compare your results for this test with you results for unhydrolyzed starch in step 4 of this experiment. Questions 1. According to the results of each part of the experiment, identify your unknown and explain your reasoning. 2. Compare the results you obtained for the Fehling's test of sucrose to the Fehling's test of hydrolyzed sucrose. Explain your results. 3. Compare the results you obtained for the Fehling's test of starch to the Fehling's test of hydrolyzed starch. Explain your results. 4. Compare the results you obtained for the iodine test of starch to the iodine test of hydrolyzed starch. Explain your results. 5. What is meant by the term "reducing sugar"? 6. What is the purpose of testing water in the Seliwanoff's test and the iodine test? 7. Draw the ring structures for α-D-fructose and for β-D-fructose. 8. An unknown carbohydrate gave a red precipitate when tested with Fehling's reagent, turned red when reacted with Seliwanoff's reagent, and quickly gave a red precipitate when reacted with Barfoed's reagent. What conclusions can be made about this carbohydrate? 9. What test could be used to differentiate between sucrose and lactose? Explain. 10. What test could be used to differentiate between glucose and starch? Explain. 11. What test could be used to differentiate between glucose and fructose? Explain. 12. Why don't all of the disaccharides undergo fermentation with yeast?
quotesdbs_dbs5.pdfusesText_9[PDF] hydroxychloroquine prophylaxis dosage for covid 19
[PDF] hyperbole maths seconde corrigé
[PDF] hyperbole maths seconde corrigé 2010
[PDF] hyperbole maths seconde corrigé 2014 pdf
[PDF] hyperref latex
[PDF] hypertonic animal cell
[PDF] hypertonic isotonic hypotonic definitions
[PDF] hypertonic isotonic hypotonic examples
[PDF] hypertonic isotonic hypotonic fluids
[PDF] hypertonic isotonic hypotonic iv solutions
[PDF] hypertonic isotonic hypotonic solution
[PDF] hypertonic isotonic or hypotonic
[PDF] hypertonic iv solutions
[PDF] hypertonic or hypotonic for dehydration
