 AUXINT-INDUCED WATER UPTAKE BY AVENA COLEOPTILE
AUXINT-INDUCED WATER UPTAKE BY AVENA COLEOPTILE
This is apparently small and not detectable by the present methods of meas- urement. When sections are placed in hypertonic solution their cells are rapidly
 Effects of Hypertonic Solution on Action Potential and Input
Effects of Hypertonic Solution on Action Potential and Input
19 Jun 1982 Exposing the muscle cell to hypertonic solution may induce cell dehydration and hence changes in the ion concentrations of myoplasm ...
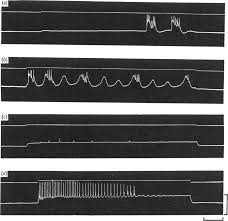 On the nature of the oscillations of the membrane potential (slow
On the nature of the oscillations of the membrane potential (slow
agents indicating that muscarinic stimulants produced their effects by acting directly on the smooth muscle cell. 5. In hypertonic solution slow waves occurred
 Cell volume variation under different concentrations of saline
Cell volume variation under different concentrations of saline
of hemoglobin from red cells it is then reasonable to assume that this level of hypertonic NaCl solutions could provoke cellular damage. Keeping in mind
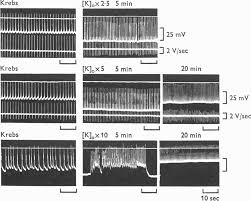 3. When the muscles were perfused with hypertonic solution
3. When the muscles were perfused with hypertonic solution
properties. They concluded that the smooth muscle cells of the portal vein could be classified as visceral smooth muscle from the electrical properties.
 Effect of Osmosis on Gummy Bears in Salt Solutions
Effect of Osmosis on Gummy Bears in Salt Solutions
In comparison to the cell's (or item's) concentration a solution can be isotonic
 III. Alterations of cell volume in extremely hypotonic the cells
III. Alterations of cell volume in extremely hypotonic the cells
volume changes in hypotonic solutions is closely related to the study of. "osmotic" hwemolysis. The methods available for the measurement of red cell volume in.
 On the nature of the oscillations of the membrane potential (slow
On the nature of the oscillations of the membrane potential (slow
Hypertonic solution. In hypertonic solution the membrane potential was All records from the same cell in hypertonic solution 35° C. Records (a) and (b) ...
 Impairment of Red Cell Transit through the Canine Lungs following
Impairment of Red Cell Transit through the Canine Lungs following
ABSTRACT. Rapid injections of hypertonic sucrose sodium chloride
 Influence of Ripening and Turgor on the Tensile Properties of Pears
Influence of Ripening and Turgor on the Tensile Properties of Pears
concentrated than the cell sap (hypertonic solutions). When similar tissue osmotic solution. The slices were vacuum infiltrated and incu- bated in the ...
 BIOL 347L Laboratory Three
BIOL 347L Laboratory Three
A Hypertonic solution has more solute (so LESS water) than the cell. A cell placed in this solution will give up water (osmosis) and shrink. A Hypotonic
 Effects of Hypertonic Solution on Action Potential and Input
Effects of Hypertonic Solution on Action Potential and Input
19-Jun-1982 to be directly related to the development of cell dehydration. The hypertonic solutions produced a slight increase in the resting potential.
 3. When the muscles were perfused with hypertonic solution
3. When the muscles were perfused with hypertonic solution
properties. They concluded that the smooth muscle cells of the portal vein could be classified as visceral smooth muscle from the electrical properties.
 On the nature of the oscillations of the membrane potential (slow
On the nature of the oscillations of the membrane potential (slow
agents indicating that muscarinic stimulants produced their effects by acting directly on the smooth muscle cell. 5. In hypertonic solution slow waves
 Introduction Fixation is an attempt at stabilizing biological systems
Introduction Fixation is an attempt at stabilizing biological systems
Tonicity. A solution is said to be isotonic with a cell if the cell neither swells nor shrinks when immersed in it. Hypertonic solutions cause shrinkage
 Ovid: Rebound Swelling of Astroglial Cells Exposed to Hypertonic
Ovid: Rebound Swelling of Astroglial Cells Exposed to Hypertonic
hypertonic solutions containing radiolabeled mannitol and its cellular uptake was determined. Results: Hypertonic mannitol exposure produced initial cell
 SICKLING PHENOMENON PRODUCED BY HYPERTONIC
SICKLING PHENOMENON PRODUCED BY HYPERTONIC
crose and uirea. The sickling phenomenon was also elicited in patients with sickle-cell anemia by hypertonic solutions of mannitol and sucrose.
 osmosis-in-onion-cells.pdf
osmosis-in-onion-cells.pdf
When discussing the way solutions separate by selective permeability the terms isotonic
 Untitled
Untitled
Water will flow into the cell causing it to swell or burst. Period: Biology. Osmosis. Isotonic. Solution. X. Date: Hypotonic Hypertonic. Solution Solution.
 Electrophysiological effects of osmotic cell shrinkage in rat
Electrophysiological effects of osmotic cell shrinkage in rat
06-May-2010 Insulin release was measured using intact islets by radioimmunoassay. exposure to a 33% hypertonic bath solution resulted in an initial ...
 Cell Diffusion & Permeability Lab - Stanford University
Cell Diffusion & Permeability Lab - Stanford University
Key Concepts: The prefix hyper- refers to “high” as in hypertension (high blood pressure) A hypertonic solution has a higher amount of solute (the solid that is being dissolved) and a lower amount of solvent (the liquid that is dissolving the solute)
 What happens to RBC in hypertonic solution? – Sage-Advices
What happens to RBC in hypertonic solution? – Sage-Advices
Hypertonic Solution Hypertonic: The solution has a higher concentration of solutes and a lower concentration of water than inside the cell (High solute; Low water)? Result: Water moves from inside the cell into the solution: Cell shrinks (Plasmolysis)! • Osmosis Animations for isotonic hypertonic and hypotonic solutions shrinks
 Lab 3: Osmosis and Diffusion - Montana State University Billings
Lab 3: Osmosis and Diffusion - Montana State University Billings
membrane a hypotonic solution will cause a cell to swell from the osmotic uptake of water Conversely if a cell is placed in a solution with a high particle (low water) concentration relative to the cell that cell will lose water The latter cell is in a hypertonic solution defined as a solution that will make a cell shrink because of the
 306 Isotonic Hypotonic & Hypertonic Notes
306 Isotonic Hypotonic & Hypertonic Notes
Hypotonic solutions are used when the cell is dehydrated and fluids need to be put back intracellularly This happens when patients develop diabetic ketoacidosis (DKA) or hyperosmolar hyperglycemia Important: Watch out for depleting the circulatory system of fluid since you are trying to push extracellular fluid into the cell to re-hydrate it
 Searches related to hypertonic solution cell filetype:pdf
Searches related to hypertonic solution cell filetype:pdf
Cells hypertonic to their surrounding solutions cause water to move into the cell and cause it to expand The cell has a higher number of particles (solutes) dissolved in it than the solution outside of the cell membrane This causes turgor pressure in plants that make the plants rigid for support
What happens to a cell in a hypertonic solution?
- Hypertonic solutions cause cells to shrivel and shrink in size, which can cause problems and inhibit proper cell functioning. When solutions surrounding cells are hypertonic, this will cause the organism to become dehydrated, which can lead to problems such as organ failure. How does tonicity affect cells?
What is the difference between a hypertonic solution and a hypotonic solution?
- A hypertonic solution has high osmotic pressure, whereas a hypotonic solution has low osmotic pressure. The concentration of solute is more in hypertonic solution than the hypotonic fluid. The concentration of solvent is low in hypertonic and high in hypotonic. Is hemolysis good or bad?
What are some common hypertonic solutions?
- Common examples of hypertonic solutions are D5 in 0.9% normal saline and D5 in lactated ringers. The administration of hypertonic solutions should be monitored extremely closely, as they can quickly lead to fluid overload. Can blood cells burst? Red blood cell lysis is more commonly known as hemolysis, or sometimes haemolysis.
What are the effects of a hypertonic solution on plant cells?
- Hypertonic solutions make plant cells lose water. Hypertonic solutions have a higher solute concentration. When plant cells are placed in such solutions, water will move from inside the plant cell to the outside of the cell, resulting in the shrinking of the cell (the cell is said to be plasmolyzed). This occurs because of osmosis.
Lab 4: Osmosis and Diffusion
The plasma membrane enclosing every cell is the boundary that separates the cell from its external environment. It is not an impermeable barrier, but like all biological membranes, is selectively permeable, controlling which molecules move into and out of the cell. For exam ple, nutrients enter the cell and waste products of metabolism leave. The cell takes in oxygen for respiration, and expels carbon dioxide. Th e membrane also regulates the concentration of ions by transporting them one way or the other across the plasma membrane. This regulation of every interaction with environment allows cells to maintain homeostasis, a steady internal state in changing conditions.Diffusion and passive transport:
Molecules are in constant m
otion, moving around randomly (Brownian movement). One result of this random motion is diffusion, the net movement of molecules from an area where their concentration is high to an area where their concentration is lower. This is a spontaneous process, requiring no inp ut of energy. In a liquid, a solute (dissolved substance) will diffuse in a solvent (dissolving agent, mo st often water in biological systems) and eventually become uniformly distributed. If a membrane separates the region of high concentration from the region of low concentration, the principle is the same. If a substance is more concentrated on one side of a membrane than on the other, it diffuses across the membrane from the region of higher concentration to the region of lower concentration, as long as th e membrane is permeable to that substance. This is called passive transport, because it requires no energy to make it happen. Much of the movement of molecules across the cell membrane occurs this way. By comparison, active transport goes against the concentration gradient, and requires the input of energy. Remember that in either case, cell membranes are selectively permeable, and not all substances m ove across freely. For example, the cell typically retains the large organic molecules it synthesizes. Water is one molecule that easily crosses the cell membrane. The net diffusion of water through a selectively permeable membrane from the side of high water concentration to the side of low water concentration is termed osmosis. The higher the concentration of solute (dissolved particles), the lower the concentration of free water molecules. What implications does osmosis have for cells? When a cell is placed in a solution in which the concentration of all solute particles is lower than the cell (and therefore, the concentration of water is higher), then water will move into the cell. Such a solution is called hypotonic. If the solute molecules are unable to pass through the cell membrane, a hypotonic solution will cause a cell to swell from the osmotic uptake of water. Conversely, if a cell is placed in a solution with a high parti cle (low water) concentration relative to the cell, that cell will lose water. The latt er cell is in a hypertonic solution, defined as a solution that will make a cell shrink because of the osmotic loss of water. A cell in an isotonic solution will have no net water uptake or loss. Note that each of these terms is relative to the inside of the cell. 1Brownian Movement
Place a drop of diluted India ink (a suspension of particles) on a cle an slide, add a coverslip, and examine with your microscope using high power and reduced illumination. Focus carefully until you see the entire field of jiggling part icles. The India ink particles "bombard" each other and are being "bombarded" by water molecules. The same energy that produces the movement of the ink particles causes diffusion of molecules. When a gradient exists, if there is no membrane blocking particle movement, diffusion will ultimately result in a uniform distribution. Place about 50 ml of water in a small beaker. Set the beaker on a white piece of paper at your table for a few minutes, until you are convinced that there are no more 'currents' in the beaker. Drop ONE drop of Methylene blue into th e beaker. Immediately record the appearance of the beaker, with respect to the concentration ofMethylene blue (the blue color).
After 3 minutes, record the appearance of the beaker again, with respect to the concentration of Methylene blue. Has there been a change? What might have caused the change?Diffusion of Water Across Cell Membranes: Osmosis
Consider a hypothetical animal cell with a composition of 10% protein and 90% water in an environment of 100% water (pure water). Remember the definition of diffusion. Water is more concentrated outside the cell, so it will move into the cell (from100% concentration to 90% concentration). In this case, the protein molecules are too
large to pass out of the cell membrane. If this movement of water (osmosis) continues unchecked, the cell may burst like a balloon. Plant cells have a rigid cell wall that prevents them from bursting. They become firm or turgid under the above conditions (that's why plants wilt from a lack of water). You looked at plant cells in under different saline concentrations last week.Osmosis Experiment
Sometimes, we use non-living models to study living systems. In this experiment, dialysis tubing is used to represent a differentially permeable membrane. When you make up a dialysis bag, think of it as a simplified cell. 2 Obtain three pieces of equal length of dialysis tubing, and several leng ths of string. Fold over one end of the tubing and tie it closed with the stri ng (you may also simply tie a knot in one end, but be careful not to put small rips in the tubing). To each tube, add 5 ml of 30% sucrose solution. Then, squeeze the bag gently to remove excess air, fold over the top, and tie it off with string. The bag should not be tight or turgid. Leave some slack in the bag as room for expansion, but get the air out. Briefly rinse the bags in running tap water, and then gently dry them on paper towels. Carefully weigh each bag to the nearest and record the results below. BE SURE TOKEEP TRACK
OF WHICH BAG IS WHICH! After weighing, place one bag in each of three labeled beakers containing: (1) tap water, (2) 30% sucrose, (3) 60% sucros e. Allow the bags to remain undisturbed for thirty minutes. Then, remove the bags, quickly rinse and dry and reweigh. Again, record the results below.Table 1. Weight (g) of dialysis bags
Tap Water 30% Sucrose 60% Sucrose
Weight at Time 0 min
Weight at Time 30
minChange in Weight
Which solution is hypotonic to the 'cell'? ___________________________ Which solution is hypertonic to the 'cell'? ___________________________ What would have happened in the following three beakers if the cell were filled with tap water instead of 30% sucrose solution?Tap Water 30% Sucrose 60% Sucrose
3 Diffusion across a differentially permeable membrane: Dialysis Dialysis is the diffusion of solute molecules across a differentially permeable membrane. The cell membrane is differentially permeable. Thus, through dialysis, certain substances may enter a cell, and certain metabolic products, including wastes, may leave. Depending on the permeability of a membrane, small solute molecules may pass through, while larger molecules are held back. Utilizing this principle, it is through dialysis that artificial kidney machines remove the smaller waste particles from the human bloodstream. The following experiment demonstrates the separation of different-sized molecules by dialysis. The two molecules used are starch (a large molecule) and sodium chloride (salt, a small molecule). In order to determine the presence of each of these molecules, we must be able to test for them.Sodium chloride (NaCl) plus silver nitrate (AgNO
3 ) produces a dense white precipitate. So, if we add silver nitrate to a solution and a precipita te forms, we may conclude that sodium chloride is present. Starch plus iodine produces a blue-black color. So, if we add iodine to a solution which then turns blue-black, we may conclude that starch is present. Add 2 drops of sodium chloride and 2 drops of silver nitrate to a culture tube. Record your results. As a control, in a second tube, add 2 drops of tap water and 2 drops of silver nitrate.Record your results.
Add 2 drops of starch solution and 2 drops of iodine to a culture tube.Record your
results. Again, as a control, add 2 drops of tap water to another tube, and 2 drops of iodine.Record your results.
Now you have seen what positive and negative tests for both sodium chloride and starch look like, so you are ready to proceed with an experiment to test the permeability of a membrane. Obtain another length of dialysis tubing and tie off one end like you di d in the osmosis experiment. Fill the bag with 2 ml of starch solution, and 2 ml of sodium chloride solution. Gently squeeze the air out, and tie off the top. Ri nse the cell off in tap water, and briefly place it on a paper towel while you perform the control test below. 4Control
Fill a beaker with tap water, and then place 2 drops from the beaker into each of two wells on a spot plate. Test one well with 2 drops of silver nitrate, and the other with 2 drops of iodine. Record your results below. (You could just write the results you got above here - there is no need to do it again.) Beaker water - silver nitrate test iodine test (time 0 min)Experiment
Now, place the 'cell' in the beaker, and record the time __________:_________ After 30 minutes, remove the cell and place it on a paper towel. Place two drops from the beaker into each of two wells on a spot plate. Test one well with 2 drops of silver nitrate, and the other with two drops of iodine. Record your res ults below (+/-Beaker water - silver nitrate test iodine test
What can you conclude from this experiment?
Why did you do the control?
Osmotic Potential of Plant Cells
As you saw last week with the Elodea leaf, living cells have some amount of water inside them, and some amount of dissolved substance (solute: sugars, salts, proteins). In thi s part of the lab, you will measure the amount of water either taken up or lost from living plant cells (cells of potato tubers), and infer the proportion of the cytoplasm that is water, and the proportion that is solute.Procedure:
Skin a potato and cut the tuber into small cubes (approximately 1cm each). You will need approximately 40 cubes. Divide the cubes into 4 groups of 10. Each group will b e immersed for 30 minutes in a different solution. The four solutions you will use are:A. distilled water (water concentration 100%)
5B. 2% saline solution (water concentration 98%)
C. 4% saline solution (water concentration 96%)
D. 8% saline solution (water concentration 92%)
Decide which of your four groups will go into each solution. Blot the c ubes dry with a paper towel, and place them on a weighing boat (dry it first too if it is moist) and weight them to the nearest 0.01g. Record the initial weight (t=0) on Table 2 bel ow. Place each group of cubes in their appropriate solutions, and wait 30 minutes (you might be collecting data from the other two experiments during this time). At the end of 30 minutes, remove the groups and blot them dry again (being sure to keep track of which group is which). Record the weight after 30 minutes in the table below. In order to account for different initial weights, you need to calculate the percent change in mass for each of your four groups. To calculate the percent change, use t he formula: % change = (weight after 30 minutes - initial weight)/initial weight X 100Record the % change in the table.
Table 2. Change in weight in potato tissue
Solution Initial Weight (t=0) Final Weight (t=30) % Change0% salt
2% salt
4% salt
8% salt
Which group had the least amount of change in mass? Which group had the greatest amount of gain in mass?Which group had the greatest loss of mass?
Which group was in the most hypertonic solution? The most hypotonic? What can you infer about the internal tonicity of potato tuber cells? Approximately how much solute and how much water are present? When you are done with the lab, open all the 'cells', pour the conte nts down the drain, dispose of the tubing and potato cubes, rinse and dry all your equipment, and wipe off your lab bench. 6quotesdbs_dbs17.pdfusesText_23[PDF] hypertonic solution definition biology quizlet
[PDF] hypertonic solution enema
[PDF] hypertonic solution example
[PDF] hypertonic solution iv
[PDF] hypertonic solution uses
[PDF] hypertonic solutions
[PDF] hypothesis of online food ordering system
[PDF] hypothyroidism icd 10
[PDF] hypotonic hypertonic isotonic worksheet
[PDF] hypotonic iv solution
[PDF] hypotonic solution cell
[PDF] hypotonic solution definition biology
[PDF] hypotonic solution example
[PDF] hypotonic solution for dehydration
