 CDC
CDC
Vaccines in the Child and Adolescent Immunization Schedule*. Vaccine. Abbreviation(s). Trade name(s). COVID-19†. 1vCOV-mRNA. Comirnaty®/Pfizer-.
 CDC
CDC
All vaccines included in the adult immunization schedule except PPSV23 RZV
 2023 Recommended Immunizations for Children from Birth Through
2023 Recommended Immunizations for Children from Birth Through
Both on the brand of Hib or of COVID-19 vaccine used. age who are getting an influenza doses should be separated by at least rotavirus vaccine used for. (flu)
 CDC
CDC
Vaccines in the Child and Adolescent Immunization Schedule*. Vaccine. Abbreviation(s). Trade name(s). COVID-19†. 1vCOV-mRNA. Comirnaty®/Pfizer-.
 Table 1: Summary of WHO Position Papers - Recommendations for
Table 1: Summary of WHO Position Papers - Recommendations for
Its purpose is to assist planners to develop an appropriate immunization schedule. Health care workers should refer to their national immunization schedules.
 Interim 2023-24 COVID-19 Immunization Schedule for Persons 6
Interim 2023-24 COVID-19 Immunization Schedule for Persons 6
Vaccine type: mRNA - Do NOT use any previously available Moderna COVID-19 vaccine products. Age. COVID-19 Vaccination History†. (regardless of COVID-19 vaccine
 Recommended Immunization Schedule for Children and
Recommended Immunization Schedule for Children and
The use of a combination vaccine generally is preferred over separate injections of its equivalent component vaccines. Vaccination providers should consult the
 2016 Combined Recommended Immunization Schedule for Persons
2016 Combined Recommended Immunization Schedule for Persons
The figure below provides catch-up schedules and minimum intervals between doses for children whose vaccinations have been delayed. A vaccine series does not
 Kenya National Immunization Policy Guidelines 2023
Kenya National Immunization Policy Guidelines 2023
5.2 Immunization schedule and vaccination of special groups. 18. 5.2.1 Routine Immunization Schedule in Kenya - 2023. 19. 5.2.2 Vaccination of Special Groups.
 2021 Recommended Child and Adolescent Immunization Schedule
2021 Recommended Child and Adolescent Immunization Schedule
Download the CDC Vaccine Schedules App for providers at www.cdc.gov/vaccines/schedules/hcp/schedule-app.html. CS310020-A. Page 2. These recommendations must be
 Recommended Child and Adolescent Immunization Schedule
Recommended Child and Adolescent Immunization Schedule
17-Feb-2022 The table below provides catch-up schedules and minimum intervals between doses for children whose vaccinations have been delayed. A vaccine ...
 Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule
17-Feb-2022 All vaccines included in the adult immunization schedule except pneumococcal ... Download the CDC Vaccine Schedules app for providers at.
 COVID-19 Vaccine Interim COVID-19 Immunization Schedule for 6
COVID-19 Vaccine Interim COVID-19 Immunization Schedule for 6
22-Aug-2022 The table below provides guidance for COVID-19 vaccination schedules based on age and medical condition. Scheduling considerations include:.
 Combined Recommended Immunization Schedule for Persons
Combined Recommended Immunization Schedule for Persons
2022 Recommended Immunizations for Children from Birth Through 6 Years Old getting an influenza (flu) vaccine for the first time and for some other ...
 Recommended Child and Adolescent Immunization Schedule
Recommended Child and Adolescent Immunization Schedule
17-Feb-2022 The table below provides catch-up schedules and minimum intervals between doses for children whose vaccinations have been delayed. A vaccine ...
 2022 Recommended Immunizations for Children 7-18 Years Old
2022 Recommended Immunizations for Children 7-18 Years Old
These shaded boxes indicate the vaccine is recommended for children with certain health or lifestyle conditions that put them at an increased risk for serious
 Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule
17-Feb-2022 Download the CDC Vaccine Schedules app for providers at www.cdc.gov/vaccines/schedules/hcp/schedule-app.html. Scan QR code for access to online ...
 Redirect for Interim COVID-19 Immunization Schedule for Ages 5+
Redirect for Interim COVID-19 Immunization Schedule for Ages 5+
COVID-19 Immunization Schedule for Ages 5+. Similar content can be found at the following URL. Please take a moment to update your bookmark:.
 Recommended Immunization Schedule for Adults Aged 19 Years or
Recommended Immunization Schedule for Adults Aged 19 Years or
All vaccines included in the adult immunization schedule except 23-valent pneumococcal polysaccharide and zoster vaccines are covered by the Vaccine Injury
 At-a-Glance COVID-19 Vaccination Schedule for Most People
At-a-Glance COVID-19 Vaccination Schedule for Most People
2 days ago COVID-19 Vaccination Schedule for Most People. (People who are NOT Moderately or Severely Immunocompromised). People ages 6 months through.
 Advisory Committee on Immunization Practices Recommended
Advisory Committee on Immunization Practices Recommended
The 2022 adult immunization schedule summarizes ACIP recommendations including several changes to the cover page tables and notes from the 2021 immuni-zation schedule † In addition the 2022 adult immunization schedule provides an appendix that lists the contraindications to and precautions for all routinely recommended vaccines in the
 COVID-19 Vaccine Interim COVID-19 Immunization Schedule for 6
COVID-19 Vaccine Interim COVID-19 Immunization Schedule for 6
Interim COVID-19 Immunization Schedule for Persons 6 Months of Age and Older The following tables provide guidance for COVID-19 vaccination schedules based on age and medical condition and vaccine composition Table 1a Moderna: Immunization Schedule for Children 6 Months through 17 Years of Age Age *
 2023-24 School Year New York State Immunization Requirements
2023-24 School Year New York State Immunization Requirements
New York State Immunization Requirements for School Entrance/Attendance1 NOTES: All children must be age-appropriately immunized to attend school in NYS The number of doses depends on the schedule recommended by the Advisory Committee on Immunization Practices (ACIP) Intervals between doses of vaccine must be in accordance
 NH SIMPLIFIED IMMUNIZATION SCHEDULE
NH SIMPLIFIED IMMUNIZATION SCHEDULE
NH SIMPLIFIED IMMUNIZATION SCHEDULE (BIRTH - 18 YEARS) ANNUAL FLU VACCINE FOR EVERYONE 6 MONTHS AND OLDER BIRTH 2 MONTH 4 MONTH 6 MONTH 12-15 MONTH 18 MONTH Hep B1 Minimum Interval 4 weeks Hep B2 16 weeks from dose 1 and 8 weeks from dose 2 and ? 24 wks of age Hep B3 DTaP1DTaP2DTaP3 Minimum Interval 6 months DTaP4 Minimum age 12 months
 Searches related to immunization schedule filetype:pdf
Searches related to immunization schedule filetype:pdf
Allina Health’s Immunization Schedule DTaP*-IPV MMR* VAR* Vaccines and Brands diphtheria tetanus and acellular pertussis + hepatitis B + inactivated poliovirus vaccine (Pediarix®) DTaP-HepB- IPV pneumococcal 13-valent conjugate vaccine (Prevnar 13™) PCV13 haemophilus influenza type b conjugate vaccine (PedvaxHIB®) Hib
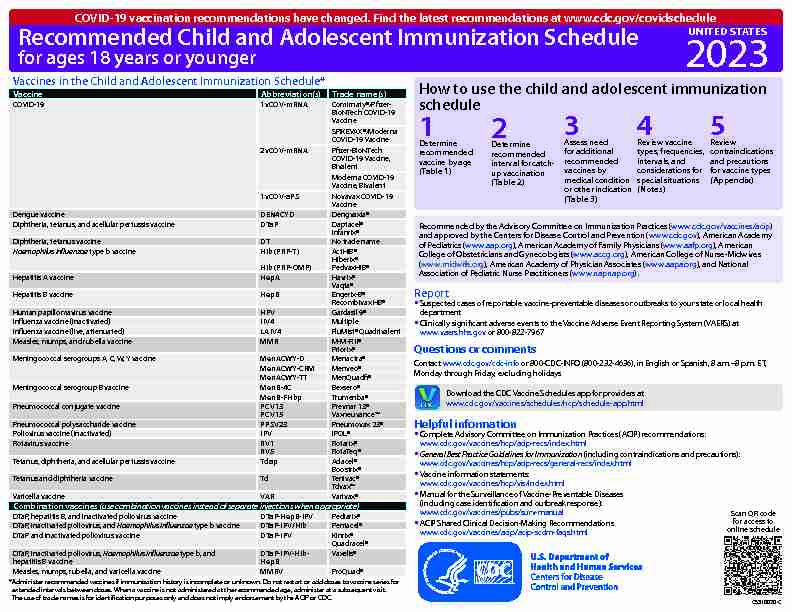 Recommended Child and Adolescent Immunization Schedule for ages 18 years or younger
Recommended Child and Adolescent Immunization Schedule for ages 18 years or younger How to use the child and adolescent immunization
schedule Recommended by the Advisory Committee on Immunization Practices (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Control and Prevention (www.cdc.gov), American Academy of Pediatrics (www.aap.org), American Academy of Family Physicians (www.aafp.org), American College of Obstetricians and Gynecologists (www.acog.org), American College of Nurse-Midwives (www.midwife.org), American Academy of Physician Associates (www.aapa.org), and National Association of Pediatric Nurse Practitioners (www.napnap.org).UNITED STATES
2023Vaccines in the Child and Adolescent Immunization Schedule*
VaccineAbbreviation(s)Trade name(s)
COVID-191vCOV-mRNAComirnaty®/Pflzer-
BioNTech COVID-19
Vaccine
SPIKEVAX®/Moderna
COVID-19 Vaccine
2vCOV-mRNAPflzer-BioNTech
COVID-19 Vaccine,
Bivalent
Moderna COVID-19
Vaccine, Bivalent
1vCOV-aPSNovavax COVID-19
Vaccine
Dengue vaccineDEN4CYDDengvaxia®
Diphtheria, tetanus, and acellular pertussis vaccineDTaPDaptacel®Infanrix®
Diphtheria, tetanus vaccineDTNo trade name
Haemophilus influenzae type b vaccineHib (PRP-T)
Hib (PRP-OMP)
ActHIB®
Hiberix®
PedvaxHIB®
Hepatitis A vaccineHepAHavrix®
Vaqta®
Hepatitis B vaccineHepBEngerix-B®
Recombivax HB®
Human papillomavirus vaccineHPVGardasil 9®
Inuenza vaccine (inactivated)IIV4Multiple
Inuenza vaccine (live, attenuated)LAIV4FluMist® Quadrivalent Measles, mumps, and rubella vaccineMMRM-M-R II®Priorix®
Meningococcal serogroups A, C, W, Y vaccineMenACWY-DMenactra®MenACWY-CRMMenveo®
MenACWY-TTMenQuadfl®
Meningococcal serogroup B vaccineMenB-4CBexsero®MenB-FHbpTrumenba®
Pneumococcal conjugate vaccinePCV13
PCV15Prevnar 13®
Vaxneuvance
Pneumococcal polysaccharide vaccinePPSV23Pneumovax 23®Poliovirus vaccine (inactivated)IPVIPOL®
Rotavirus vaccineRV1
RV5Rotarix®
RotaTeq®
Tetanus, diphtheria, and acellular pertussis vaccineTdapAdacel®Boostrix®
Tetanus and diphtheria vaccineTdTenivac®
Tdvax
Varicella vaccineVARVarivax®
Combination vaccines (use combination vaccines instead of separate injections when appropriate) DTaP, hepatitis B, and inactivated poliovirus vaccineDTaP-HepB-IPVPediarix® DTaP, inactivated poliovirus, and Haemophilus influenzae type b vaccineDTaP-IPV/HibPentacel® DTaP and inactivated poliovirus vaccineDTaP-IPVKinrix®Quadracel®
DTaP, inactivated poliovirus, Haemophilus influenzae type b, and hepatitis B vaccineDTaP-IPV-Hib-
HepBVaxelis®
Measles, mumps, rubella, and varicella vaccineMMRVProQuad®Administer recommended vaccines if immunization history is incomplete or unknown. Do not restart or add doses to vaccine series for
extended intervals between doses. When a vaccine is not administered at the recommended age, administer at a subsequent visit.
The use of trade names is for identication purposes only and does not imply endorsement by the ACIP or CDC.
Report
Suspected cases of reportable vaccine-preventable diseases or outbreaks to your state or local health
department Clinically signiflcant adverse events to the Vaccine Adverse Event Reporting System (VAERS) at www.vaers.hhs.gov or 800-822-7967Questions or comments
Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Spanish, 8 a.m.-8 p.m. ET,
Monday through Friday, excluding holidays
Helpful information
Complete Advisory Committee on Immunization Practices (ACIP) recommendations: General Best Practice Guidelines for Immunization (including contraindications and precautions):Vaccine information statements:
www.cdc.gov/vaccines/hcp/vis/index.html Manual for the Surveillance of Vaccine-Preventable Diseases (including case identication and outbreak response): www.cdc.gov/vaccines/pubs/surv-manual ACIP Shared Clinical Decision-Making RecommendationsDetermine
recommended vaccine by age (TableDetermine
recommended interval for catch- up vaccination (TableAssess need
for additional recommended vaccines by medical condition or other indication (TableReview vaccine
types, frequencies, intervals, and considerations for special situations (Notes)Review
contraindications and precautions for vaccine types (Appendix) Download the CDC Vaccine Schedules app for providers atCS310020-C
Scan QR code
for access to online scheduleCOVID-19 vaccination recommendations have changed. Find the latest recommendations at www.cdc.gov/covidschedule
These recommendations must be read with the notes that follow. For those who fall behind or start late, provide catch-up vaccination at the earliest opportunity as indicated by the green bars.
To determine minimum intervals between doses, see the catch-up schedule (Table 2).VaccineBirth1 mo2 mos4 mos6 mos9 mos12 mos15 mos18 mos19-23 mos2-3 yrs4-6 yrs7-10 yrs11-12 yrs13-15 yrs16 yrs17-18 yrs
Hepatitis B (HepB)1
dose----- 2 dose -----W---------------------------- 3 dose ----------------------------Rotavirus (RV): RV1 (2-dose series),
RV5 (3-dose series)
dose2 doseSee NotesDiphtheria, tetanus, acellular pertussis
(DTaP <7 yrs) dose2 dose3 dose----- 4 dose ------5 doseHaemophilus infiuenzae type b (Hib)1
dose2 doseSee Notes or 4 dose,See Notes
Pneumococcal conjugate
(PCV13, PCV15) dose2 dose3 dose----- 4 dose -----Inactivated poliovirus
(IPV <18 yrs) dose2 dose---------------------------- 3 dose ----------------------------4 dosequotesdbs_dbs7.pdfusesText_5[PDF] immunofluorescence protocol tissue
[PDF] immunologie approfondie cours pdf
[PDF] immunologie cours pdf 2eme année biologie
[PDF] immunologie cours pdf s5
[PDF] immunologie fondamentale cours pdf
[PDF] immunologie generale cours pdf
[PDF] immunologie medicale cours pdf
[PDF] impact factor pdf
[PDF] impact font
[PDF] impact font meme
[PDF] impact investing association
[PDF] impact investing in affordable housing in india
[PDF] impact investing in emerging markets
[PDF] impact investing metrics
