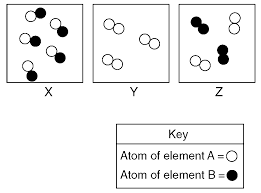
 HW # 1: Solution Name ROW ____ PD ____
HW # 1: Solution Name ROW ____ PD ____
Feb 28 2017 A) a compound. B) an element. C) a mixture. D) a substance. 7. Bronze contains 90 to 95 percent copper and 5 to ...
 Heterogeneous Mixture - Homogeneous Mixture Worksheet-Answer
Heterogeneous Mixture - Homogeneous Mixture Worksheet-Answer
Describe the following as an element a compound
 2020-09-14 16:50
2020-09-14 16:50
Sep 14 2020 Classify each of the following as an element
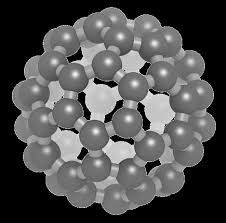 We use materials every single day – our cotton sheets the springs
We use materials every single day – our cotton sheets the springs
All the properties of elements compounds and mixtures can be classified into two different types: chemical properties and physical properties. Physical
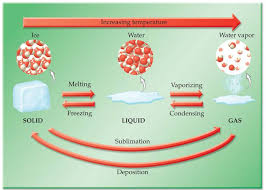 EXAMPLE EXERCISE 4.1 Change of Physical State
EXAMPLE EXERCISE 4.1 Change of Physical State
(b) Copper oxide is a compound of the elements copper and oxygen. (c) Malachite ore is a heterogeneous mixture of copper and other substances. (d) Bronze alloy
 Untitled
Untitled
If the material is a pure substance further classify it as either an element or compound in the right column. or a heterogeneous mixture. 1. Sand.
 Untitled
Untitled
Classify each diagram as: pure substance - element pure substance - compound
 CLASSIFICATION OF MATTER WORKSHEET HOMOGENEOUS VS
CLASSIFICATION OF MATTER WORKSHEET HOMOGENEOUS VS
Element. Compound Mixture lead metal table salt. (NaCl). Kool-Aid drink vegetable soup oxygen gas distilled water. Concrete pure gold brass metal flat 7-Up soda.
 Elements Compounds & Mixtures Worksheet
Elements Compounds & Mixtures Worksheet
Elements: •. A pure substance containing only one kind of atom. An element is always uniform all the way through (homogeneous). An element cannot nuclear
 Chemistry: Classifying Matter Name______________________
Chemistry: Classifying Matter Name______________________
If the material is a pure substance further classify it as either an element or compound in the right column. heterogeneous mixture? Give an example of each ...
 EXAMPLE EXERCISE 4.1 Change of Physical State
EXAMPLE EXERCISE 4.1 Change of Physical State
(b) Copper oxide is a compound of the elements copper and oxygen. (c) Malachite ore is a heterogeneous mixture of copper and other substances. (d) Bronze
 Elements Compounds & Mixtures Worksheet
Elements Compounds & Mixtures Worksheet
Elements: • A pure substance containing only one kind of atom. An element is always uniform all the way through (homogeneous)
 Untitled
Untitled
matter including classification as elements compounds and mixtures
 Chemistry: Classifying Matter Name______________________
Chemistry: Classifying Matter Name______________________
substance or a mixture. If the material is a pure substance further classify it as either an element or compound in the right column.
 CLASSIFICATION OF MATTER WORKSHEET HOMOGENEOUS VS
CLASSIFICATION OF MATTER WORKSHEET HOMOGENEOUS VS
PURE SUBSTANCES VS. MIXTURES. Classify the following as pure substances (element or compound) or mixtures. 1. sodium Pure substance (E). 11
 Untitled
Untitled
Brass Alloy (Cu + Zn). 10. Oil-Vinegar Elements Compounds Homogeneous Heterogeneme ... Elements. MIXTURES. Heterog. Mix. Homos. Mix. Element. Compound-.
 Whats the Difference? - Atom Element
Whats the Difference? - Atom Element
Compound
 Tiered Activity: Elements Compounds
Tiered Activity: Elements Compounds
http://www.troup.org/userfiles/929/My%20Files/Instructional%20Strategies/tiered_elem_compound_mixtures.pdf?id=14740
 Mixtures Worksheet Answer Key
Mixtures Worksheet Answer Key
If the material is a pure substance identify it as an element or a compound. Material. Mixture. Pure Substance. Homogeneous or Heterogeneous.
 KEY - X Unit 3 Review Packet.pdf
KEY - X Unit 3 Review Packet.pdf
A mixture (is/is not)) a chemical combining of substances. 2. In a compound the (atoms/molecules) are (chemically/physically) combined so that the elements
 Is bronze a mixture or compound? How do you learn the difference?
Is bronze a mixture or compound? How do you learn the difference?
Bronze is a mixture of tin and copper They are heated to the melt point of the higher melting metal I believe and then the other is added
 Is Bronze an Element Compound or Mixture? [ANSWERED]
Is Bronze an Element Compound or Mixture? [ANSWERED]
Bronze is a mixture since it's made of two different elements (Copper and Tin) that combine without forming chemical bond Thus bronze cannot be considered a
 [PDF] Elements Compounds and Mixtures
[PDF] Elements Compounds and Mixtures
An element contains just one type of atom A compound contains two or more different atoms joined together A mixture contains two or more different
 [PDF] Elements Compounds and Mixtures
[PDF] Elements Compounds and Mixtures
An element contains just one type of atom A compound contains two or more different atoms joined together A mixture contains two or more different
 [PDF] MIXTURES ELEMENTS AND COMPOUNDS - Junta de Andalucia
[PDF] MIXTURES ELEMENTS AND COMPOUNDS - Junta de Andalucia
Steel is a homogeneous mixture however it is made from iron and carbon A pure substance is different from a homo- geneous mixture because a pure substance has
 [PDF] ELEMENTS COMPOUNDS & MIXTURES
[PDF] ELEMENTS COMPOUNDS & MIXTURES
A compound is a pure substance that consists of atoms of two or more elements joined together Compounds are formed when atoms of different elements react
 [PDF] Elements Compounds & Mixtures Worksheet
[PDF] Elements Compounds & Mixtures Worksheet
Elements Compounds Mixtures Worksheet Part 1: Read the following information on elements compounds and mixtures Fill in the blanks where necessary
 [PDF] Elements Compounds & Mixtures Notes & Practice
[PDF] Elements Compounds & Mixtures Notes & Practice
%2520compounds
 [PDF] Elements Compounds Mixtures
[PDF] Elements Compounds Mixtures
1 fév 2016 · a mixture containing a metallic element and a non-metallic element • Common alloys include ? Bronze – copper (88 ) and tin (12 )
Is a bronze an element compound or mixture?
Bronze is a mixture of two elements, copper and tin.Is bronze a element or not?
Bronze is a metal alloy that primarily contains copper and 12% tin. Other elements—such as aluminum, arsenic, manganese, phosphorus, and silicon—are also added to yield different properties. These mixtures form some of the common bronze alloys, including: Leaded bronze.Is bronze a mixture and not compound?
Brass and bronze are mixtures.- Bronze is an alloy consisting primarily of copper, commonly with about 12–12.5% tin and often with the addition of other metals (including aluminium, manganese, nickel, or zinc) and sometimes non-metals, such as phosphorus, or metalloids such as arsenic or silicon.
Elements, Compounds, and
Mixtures
Pure Substances
yA sample of matter that has definite chemical and physical properties.Elements
ypure substance that cannot be separated into simpler substance by physical or chemical means.An atom consists of a
yNucleus
protons and neutrons y electrons in space around the nucleus.The Atom
Nucleus
Electron cloud
The building blocks of
Matter
Consists of Protons (+),
Electrons (-),
and Neutrons (N).Smallest unit of an
elementthat has all of he same properties of that element.Images are from http://www.chem4kids.com
Animated images and notes from http://www.chem.purdue.edu/gchelp/atoms/elements.htmlAtoms of two or more differentelements
joined together by chemical bonds.In the animation above, water (H20) is a
compound made of Hydrogen andOxygen.
The red compound is
composed of nickel (Ni) (silver)carbon (C) (black)
hydrogen (H) (white) R[\JHQ 2 UHG
nitrogen (N) (blue)CHEMICAL COMPOUNDS
composed of atoms and so can be decomposed(broken down) to those atoms.Compounds
yMade of elements in a specific ratio that is always the same yHave their own physical and chemical properties. yCan only be separated by chemical means, not physically ySmallest particle is a moleculeA moleculeconsists of two or more atoms of
the sameelement, or differentelements, that are chemically bound together.In the animation above, two nitrogen atoms
(N + N = N2) make one Nitrogen molecule .Chemical Bonds
yMolecules
are held together by bonds yIonic bonds
yCovalent bonds
IONS y IONS are atoms or groups of atoms with a positive or negative charge. yTo tell the difference between an atom
and an ion, look to see if there is a charge in the superscript! Examples: Na Ca +2 I O 2Na Ca I O
Forming Positive & Negative
IonsA Positive ion
forms when an atom loses one or more electrons.An Negative ion
forms when an atom gains one or more electronsMg --> Mg2+(2 e-lost)F + e---> F-
IONIC BONDS
y metals (Mg) lose electrons positive ion y nonmetals (F) gain electrons negative ion yOPPOSITES ATTRACT EACH OTHER!
y positive ionIS ATTRACTED TO
negative ionCharges on Common Ions
-1-2-3+1 +2By losing or gaining e
, atom has same number of e ClCovalent Bonds
Form when two or more
atoms SHARE electronsMixtures
yA combination of twoor morepure substances that are notchemically combined. ysubstances held together by physical forces, not chemical yNo chemical change takes place yEach item retainsits properties in the mixture yThey can be separated physicallyChem4kids.com
Solutions are groups of molecules that are
mixed up in a completely even distribution.Uniform ( the same) Distribution.
Example: Sugar dissolved in Water
Images are from http://www.chem4kids.com
The substance to be dissolved.
The one doing the dissolving.
Images are from http://www.chem4kids.com
Particle sizes are in between the size of particles found in solutions and suspensions . Can be mixed and remain evenly distributed without settling out.The substances are not uniformly mixed.
Example: Sand in a glass of water.
Images are from http://www.chem4kids.com
Are heterogeneousmixtures consisting of parts
that are visible to the naked eye.Example: the ingredients in salad dressing
Substances will settleover time.
Mixtures vs. Compounds
Can you identify the following?
You will be shown a series of photos. Tell if each photo represents an item composed of an element, compound, or mixture.Review:
yAnelementcontains just one type of atom. yAcompoundcontains two or more different atoms joined together. yAmixturecontains two or more different substances that are only physically joined together, not chemically. yA mixture can contain both elements and compounds.Element, Compound, or Mixture?
Copper
Element, Compound, or Mixture?
Copper
Element, Compound, or Mixture?
Jelly Beans
Element, Compound, or Mixture?
Jelly Beans
Element, Compound, or Mixture?
Table Sugar
Element, Compound, or Mixture?
Table Sugar
Element, Compound, or Mixture?
Diamond
Element, Compound, or Mixture?
Diamond
Element, Compound, or Mixture?
TeaElement, Compound, or Mixture?
TeaElement, Compound, or Mixture?
SaltElement, Compound, or Mixture?
SaltElement, Compound, or Mixture?
Neon Gas
Element, Compound, or Mixture?
Neon Gas
Element, Compound, or Mixture?
SaladElement, Compound, or Mixture?
SaladElement, Compound, or Mixture?
Pure Water
Element, Compound, or Mixture?
Pure Water
Element, Compound, or Mixture?
Aluminum
Element, Compound, or Mixture?
Aluminum
Element, Compound, or Mixture?
Lemonade
Element, Compound, or Mixture?
Lemonade
Element, Compound, or Mixture?
Silver
Element, Compound, or Mixture?
Silver
Element, Compound, or Mixture?
SandElement, Compound, or Mixture?
Sand Notes yDetailed notes are located at: mixtures-notes-isn.pdf yFlow Chart: isn.pdfquotesdbs_dbs4.pdfusesText_8[PDF] is calling guam considered international verizon
[PDF] is chocolate chip cookie dough ice cream heterogeneous or homogeneous
[PDF] is chocolate chip ice cream a homogeneous mixture
[PDF] is chocolate chip ice cream a pure substance homogeneous or heterogeneous
[PDF] is chocolate chip ice cream homogeneous
[PDF] is cobol still used
[PDF] is concrete homogeneous or heterogeneous
[PDF] is confederate money printed on both sides
[PDF] is crypto the future of money
[PDF] is d5w hypotonic
[PDF] is design pattern a logical concept
[PDF] is dextrose bad for you
[PDF] is dextrose saline a colloid or crystalloid
[PDF] is education free in switzerland
