 สมดุลวัฏภาค Phase Equilibria
สมดุลวัฏภาค Phase Equilibria
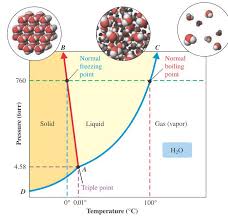
*Melting point decreases with increasing pressure (blue line) because its liquid form is more compact than its solid form. Phase Diagram of Water (H. 2. O)
 1 critical point triple point
1 critical point triple point
Thoroughly understanding the concept of sublimation is a key building block to gaining knowledge of freeze drying. As shown below on the phase diagram for water
 Calculation of a temperature–volume phase diagram of water to
Calculation of a temperature–volume phase diagram of water to
17 ก.ค. 2565 alongside 1D three-phase coexistence lines as opposed to the 0D three-phase triple points found in T–P diagrams. These varied heterogeneous ...
 Making a Splash on Mars There are trace quantities of water vapor
Making a Splash on Mars There are trace quantities of water vapor
17 มิ.ย. 2556 Above: A phase diagram of water. The 'triple point' is the temperature and pressure where all three types of water can exist at once. In the ...
 Untitled
Untitled
Part B - Phase Diagram for Water. PHASE DIAGRAMS #3. (Single Component). 12 What letter represents the triple point? 5. In your own words what is the ...
 Calculation of a temperature–volume phase diagram of water to
Calculation of a temperature–volume phase diagram of water to
three-phase coexistence points at the meetings of any three single-phase regions (so-called triple points).12. The temperature–concentration diagram for a
 Calculation of a temperature-volume phase diagram of water to
Calculation of a temperature-volume phase diagram of water to
These lines which occur at the temperatures and pressures of the triple points common in T-P phase diagrams
 PHASE DIAGRAM WORKSHEET #2 Name________________
PHASE DIAGRAM WORKSHEET #2 Name________________
(17). At what range of pressure will water be a liquid at temperatures above its normal boiling point? (18). In what phase does water exist at its triple point?
 Thermal Properties of Matter
Thermal Properties of Matter
For example the triple point of water is represented by the temperature 273.16 K and pressure 6.1110–3 Pa. (a). (b). Pressure-temperature phase diagrams for (a)
 CPY Document
CPY Document
Draw a small box around the triple point. 10. The figure shows the phase diagram of water. Answer the following questions by explicitly referring to the phase.
 Water Phase Diagram
Water Phase Diagram
7 Nov 2017 The properties of the all the known different phases of water are described. Phase diagrams. The phase diagram of water. Triple points.
 Untitled
Untitled
Phase Diagrams: Comparing H?O (left) and CO2 (right). Liquid water. (liquid) Increased pressure will (lower/raise) the melting point of water.
 1) A phase diagram is a graph of pressure vs. temperature that
1) A phase diagram is a graph of pressure vs. temperature that
d) Triple Point - indicates the temperature & pressure conditions at which the solid liquid
 The Phase Diagram of Water
The Phase Diagram of Water
24 Jan 2008 Where three lines join there is a 'triple point' when three phases ... Other phase diagrams for water are presented elswewhere [681].
 Lecture 9: Phase Transitions
Lecture 9: Phase Transitions
Here are some example phase diagrams for carbon dioxide argon and water. Figure 4. At the triple point
 Phase diagram of the TIP4P/Ice water model by enhanced sampling
Phase diagram of the TIP4P/Ice water model by enhanced sampling
We studied the phase diagram for the TIP4P/Ice water model using enhanced From each liquid-ice-ice triple point we then conduct a new Gibbs-Duhem ...
 The phase diagram of water and the magnetic fields of Uranus and
The phase diagram of water and the magnetic fields of Uranus and
pressure phase diagram of water we can specify the region where superionic water should occur The location of the triple point between ice fluid
 PHASE DIAGRAM WORKSHEET - Chemistry
PHASE DIAGRAM WORKSHEET - Chemistry
boiling point? Tatm-225 atm. (18). In what phase does water exist at its triple point? all 3 phases: Solid liquid gas. (19). How does the melting point of
 Computational thermodynamics: how to calculate phase diagrams
Computational thermodynamics: how to calculate phase diagrams
temperature pressure density composition …etc. phase boundaries critical points triple point …etc. Important in chemistry materials
 Lesson Plan: Phase Diagrams and Phase Equilibria
Lesson Plan: Phase Diagrams and Phase Equilibria
Define and explain the triple point and critical point in a phase diagram. • What are supercritical fluids? How are they useful? • Discuss the water vapor
 Phase Diagrams and the Triple Point
Phase Diagrams and the Triple Point
Phase Diagrams and the Triple Point Consider an isolated (adiabatic) container of water at 100° C This container has only water in vapor and liquid form—no air or any other substance In this container: • The vapor is in equilibrium with the liquid; that is if one watches the container the amounts of vapor and liquid do not change
 Water Phase Diagram - IDC-Online
Water Phase Diagram - IDC-Online
The phase diagram of water Density change Triple points The ice phases Phase diagrams Phase diagrams show the preferred physical states of matter at different temperatures and pressure Within each phase the material is uniform with respect to its chemical composition and physical state
 Major Features of a Phase Diagram Introduction to Chemistry
Major Features of a Phase Diagram Introduction to Chemistry
Phasediagramofwater meltingpointdecreaseswithincreasing COmeltingpointincreaseswithincreasing 2 ATER Covers~ Lifeon ater Easily Yet70 of the earth’s surface earthdependsonwater isa“universal”solvent polluted;hardtopurify wateris an anomaly“Wateristhemostremarkablesubstance Howeverwateris oftenperceivedtobeprettyordinary Wewashinwaterfish
 Phase Rule CHAPTER-6 PHASE RULE
Phase Rule CHAPTER-6 PHASE RULE
The phase diagram for the water system is shown in Fig 6 1 The phase diagram consists of 1 Curves: There are three curves OA OB and OC 2 Areas: Three curves OA OB and OC divide the diagram into three areas AOB AOC and BOC 3 Triple point: The above three curves meet at the point O and is known as triple point
 PHASE DIAGRAMS - ScienceGeeknet
PHASE DIAGRAMS - ScienceGeeknet
PHASE DIAGRAMS This is a phase-change diagram for water 1 Along LEG ‘A’ water exists as a solid (ice) and the temperature increases as HEAT energy is absorbed 2 At 0 °C a phase change begins: a) Moving from left to right along LEG ‘B’ ice is melting to form liquid water
 Searches related to phase diagram of water triple point filetype:pdf
Searches related to phase diagram of water triple point filetype:pdf
The triple point occurs at 175 5 K Sketch (not to scale) the single-component phase diagram for methanol Strategy Consider Figure 17 4 which shows a typical phase diagram for a one-component system Remember that the normal melting and boiling points correspond to a pressure of 1 atm = 1 013 bar Solution
What does the triple point on a phase diagram describe?
- Triple point is found on a phase diagram where the three lines of equilibrium between states of matter converge. The triple point is a temperature and pressure combination.
What is the triple point on a phase diagram apex?
- What is triple point in phase diagram? The triple point is the point on the phase diagram where the lines of equilibrium intersect — the point at which all three distinct phases of matter (solid, liquid, gas) coexist. ... At what temperature will a solid melt apex? At temperatures above 32°F (0°C), pure water ice melts and changes state ...
How to calculate triple point?
- There is no general formula that will allow you to determine the triple point of a substance. Ideally, for a given substance one would need an equation of state to do this. An equation of state would tell, for example, the pressure given the temperature and density.
What is a triple point diagram?
- Triple point – the point on a phase diagram at which the three states of matter: gas, liquid, and solid coexist. Critical point – the point on a phase diagram at which the substance is indistinguishable between liquid and gaseous states.
MatthewSchwartz
StatisticalMechanics,Spring2019
Lecture9:PhaseTransitions
1Introduction
Figure1.Twoofthephasesofsolidcarbon
A⎷haseisauniformstateofmatter.
wantittobe.Wewant"gas"tobethephase.Amoretechnicallyprecisedefinitionis
ofP;V;Tetc). importantareaofphysics. 1 ofthephasetransformationarerelated.2Solids,liquidsandgases
T=¡1
V@V @P T (1) gaslawPV=NkBT,weseethatforagasT=∞P=/0.Compressibilityisanexampleofanorder
=GAiscalled
thesurfacetension:2Section2Atohavinga
surface,with thesurfacetension. (e.g. water=73mN mcomparedtosay,CO2=17mN
m),butnotthelargest(mercuryhasHg=486mN
m). ofmetal. temperatureandpressure. k BT3 fora2mkBp;foramoregeneralideal
gas=kBTlnP k BT equilibriumthatwearediscussinghere). energyG.Recallthat dG=VdP¡SdT+1dN1+2dN2(2) gas,ittakestheform=kBTlnN VMelting:transitionfromsolidtoliquid.
Condensation:transitionfromgastoliquid.
Sublimation:transitionfromsolidtogas.
Nfixed.Then,=G
Nandso@
@T P =1 N@G @T P =-S N(4) infinitesimallybelowit∂G ∂T P ∂T P =-Sgas.Inapure solid=liquid=gas.3Phaseboundaries
dominantes,andNisfixed(dN=0).SinceGN=,thendG=NdandfromEq.(2),dG=
VdP-SdT,wefind
d=V NdP-SNdT(5)
whichgives V 1N1dP-S1
N1dT=V2
N2dP-S2
N2dT(6)
Thatis
dP dT=S N VN(7)Phaseboundaries5
3.1Latentheat
N.RecallthatG=H-TSso
μ=G
N=H N-TSN=·H
Natthephaseboundary.Sowecan
canalsowritetheClapeyronequationas dP dT=∞ TL·¡1
n(8) wheren=NVisthenumberdensityand
L=·H
N (9) iscalledthelatentheat.T.Since·S=H
Twe putinpermoleculetochangethephase. -286kJ molandforwatervaporis·fH=-242kJ ofvaporizationofwaterat1atm:Lvap=44kJNotethat44kJ
molK, molofenergy.That L fuse=6.0kJ thatLfuse>0sinceittakesheattomeltice. L densethantheirliquidforms.So·¡1 n&0.ThereforebyEq.(8),dP dTisgenerallyverylargeand 2Figure5.Phasediagramforwater.
dT<0.SinceL>0andT>0this mustmean·-1 n n=∞ n gas¡∞ n liquid∞ n gas=Vgas N gas=RT P(10) dP dT=PLRT2(11)
PdP=LRT2dT(12)
wecanintegratebothsidestogiveP=Cexp
¡L RT (13) wethenhaveP=Pexp
¡LR∞
T¡∞
T (14)Phaseboundaries7 molandR=8.3J mol, weget T=1T¡R
LlnPP¡1
=370.1K(15) whichisthreedegreeslower.3.2Vaporpressure
T =373K,P=1atmandL=42kJ w(P0;T0)=gas(P0;T0)(16) wmix(P;T)=w(P;T)¡kBTNsNw(17)
P0bywritingP=P0+Pweget
wmixed(P;T0)=w(P0+P;T0)¡kBT0NsNw=w(P0;T0)+P@w
@P T¡kBT0Ns
Nw(18)8Section3
Similarly,
gas(P;T0)=gas(P0;T0)+P@gas @P T (19) Now,@ @P T =V PVwNw¡Vgas
Ngas =kBT0NsNw(20)
Vismuchlarger),
sowecandropVw N wcomparedtoVgas N gas.Usingtheidealgaslaw,Vgas N gas=kBT0 P0wethenhave
(P¡P0)¡kBT0
P0 =kBT0NsNw(21)
orP=¡NsNwP0(22)
atalowervaporpressure.ClapeyronequationdP
dT=PLRT2,Eq.(11).RecallthatdP
dTistheslopeofthephaseboundary.For smallPandTwecanusedP dT=P pressure,soT=¡PRT2
P 0L=NsNwRT02
L(23) vaporizationofwaterL=42kJ mol,about1/7thofthelatentheatof vaporization.ThenT=¡8.7
558.3J
molK(273K)2 6.0kJ mol=¡16K(24)3.3Chemicalpotentialphasediagrams
whichissometimesuseful. athighT.Wealsoknowthat@ @T P =¡SN<0(25)
solidlines. bothexpansions.Tryityourself!10Section3 then@ @P T =V N=1 n>0(26) inqualitativeagreementwithEq.(14).Here"sanotherexample
Figure8.Phasediagramforsubatomicmatter
4Generalphasetransitions
∂T P changesdiscontinuouslyattheFirstorderphasetransition:∂G
∂T P changesdiscontinuouslyatthephaseboundary ∂T P tobecontinuous, nthorderphasetransition:∂nG ∂Tn P intoone,andthelatentheatvanishes. theentropyS=¡@G @T P Vis equivalenttousingV=@F @P T4.1Paramagnetism
anexampleofaphasetransition. phase. =2N F magentic=E¡TS=¡N"(27) andthefreeenergyofthedisorderedstateis F stateoccursat k BTc" ln2(29) T cisknownastheCurietemperature. transitionissecondorder. magneticfieldisavectorM,andwecandefineM=M.AboveTc,M=0exactly.AsTis
symmetryisspontaneouslybroken.4.2Criticalphenomena
properties.Generalphasetransitions13N.Thegreenregionhas
liquidandgas. ∂v=0.Afterthephasetransitionis coexistenceregion. volume,v=1 ∂v? T =0.Atthecriticalpoint,thelengthofthe ∂v2? T =0aswell:thecritical14Section4 thederivativesofPvanish,∂nP ∂vn TIt"snotjustthederivatives∂nP
∂vn T diagramintheT-vplane:Figure11.TVdiagramforwater
∂vn P =0,soT(v)isanon-analytic function. ∂vn T =∂nT ∂vn P =0,allthedimensionfulphysical andeverything surfacetension thecriticalvalues: T ^=T T c;P^=P P c;v^=v v c;n^=1 v^=n n c= c(30) n^?(T^)=1+34(1-T^)+7
4(1-T^)1/3;n^g(T^)=1+3
4(1-T^)-7
4(1-T^)1/3(32)
pointn^=1,T^(k)(1)=0.16Section4quotesdbs_dbs14.pdfusesText_20[PDF] phase diagram of water vs other substances
[PDF] phase diagram of water worksheet
[PDF] phase of a discrete signal
[PDF] phases of water
[PDF] phatic function of language examples
[PDF] phd economics
[PDF] phd economics europe
[PDF] phd in disaster management in ignou
[PDF] phe budget 2020
[PDF] phelan
[PDF] phemius pronunciation
[PDF] phenol acetic anhydride reaction mechanism
[PDF] phenol acidity
[PDF] phenol pka
