 Stomach Acid, pH, and Health
Stomach Acid, pH, and Health
14 A pH of 7 is considered neutral Below 7 pH is acidic and above 7 pH is basic The farther away from a neutral ph of 7, the stronger the acid or base Our bodies work best at a slightly alkaline pH Blood pH is generally maintained at about 7 3 pH and relies on a buffer system of minerals for proper control over pH balance
 “Buffers and Stomach Acid”
“Buffers and Stomach Acid”
acid (HCl) The pH of gastric juice is 1 5 The pH scale measures how acidic or basic a substance is An acidic solution has a high concentration of Hydrogen ions (H+) compared to a basic solution that has a high concentration of hydroxide ions (OH-) The pH scale ranges from 0 to 14 A pH of 7 is neutral A pH less than 7 is acidic A pH greater
 Buffers, pH & Gastric Acid: An Overview
Buffers, pH & Gastric Acid: An Overview
•Low pH has the effect of destroying most microorganisms entering the GIT; •Some clinical conditions may arise from defects in digestive processes, such as ulceration by Gastric HCl or diminished secretion of HCl causing Achlorhydria; •Parietal cells may secrete HCl at concentration of 160 mM (equivalent to pH of 0 8);
 Oesophageal manometry and 24-hour pH monitoring
Oesophageal manometry and 24-hour pH monitoring
PI19_0374_09 Oesophageal manometry and 24-hour pH monitoring 5 through your nose You will be asked to drink a beaker of water through a straw; this will help the tube to move through the throat and down the oesophagus You will be able to breathe normally at all times Once the tube is in place you will feel a ‘lump’ sensation in your
 PHYSIOLOGIE GASTRIQUE - Weebly
PHYSIOLOGIE GASTRIQUE - Weebly
Acidité du chyme : à pH inférieur à 3,5 l’activité motrice de l’estomac est inhibée, tandis que le duodénum se contracte vivement Ce gradient de pression arrête l’évacuation de l’estomac jusqu’à ce que le pH remonte (sécrétions alcalines biliaires et pancréatiques)
 Étude avec une sonde de pH - GiKids
Étude avec une sonde de pH - GiKids
La mesure du pH dans l’œ-sophage aide à déterminer s’il y a un reflux d’acide en provenance de l’estomac L’étude avec une sonde de pH s’effectue habituellement chez les patients qui ont des symptômes de reflux gastro-œsophagien Elle sert également à établir l’efficacité d’un traitement aux antiacides
 Symptoms That the Body Is Too Alkaline - Imune
Symptoms That the Body Is Too Alkaline - Imune
All chemicals, natural and otherwise, have a pH level, which is a measurement of hydrogen The pH scale ranges from zero to 14, with zero being purely acidic, 14 being purely alkaline and seven being neutral According to "The Complete Book of Enzyme Therapy" by Anthony J Cichoke, the human body's optimal pH range is between 7 35
 Student Exploration: pH Analysis: Quad Color Indicator
Student Exploration: pH Analysis: Quad Color Indicator
The pH scale runs from 0 to 14, with 0 representing the highest concentration of hydrogen ions Acidic substances have a pH below 7, while alkaline substances (bases) have a pH above 7 Pure water has a pH of 7 and is considered neutral The pH Analysis: Quad Color Indicator Gizmo™ allows you to find the pH of a variety of liquids
[PDF] ph et croissance bactérienne
[PDF] ph mélange acide faible base faible
[PDF] ph métrie chimie analytique
[PDF] ph métrie cours
[PDF] ph métrie définition
[PDF] ph métrie principe
[PDF] ph sanguin, H3O+, calcul
[PDF] ph suc intestinal
[PDF] Ph-Ch Masse de Cuivre dans une pièce
[PDF] phaedra act 2 scene 5
[PDF] phaedra jean racine full text
[PDF] phagocytes
[PDF] phagocytose
[PDF] phalène bouleau sélection naturelle
 Halperin Chiropractic, 634 7th Avenue, Kirkland, WA 98033
Halperin Chiropractic, 634 7th Avenue, Kirkland, WA 98033 425-822-2858 www.keithhalperin.com Page 1 of 2
Stomach Acid, pH, and Health
What's in a number?
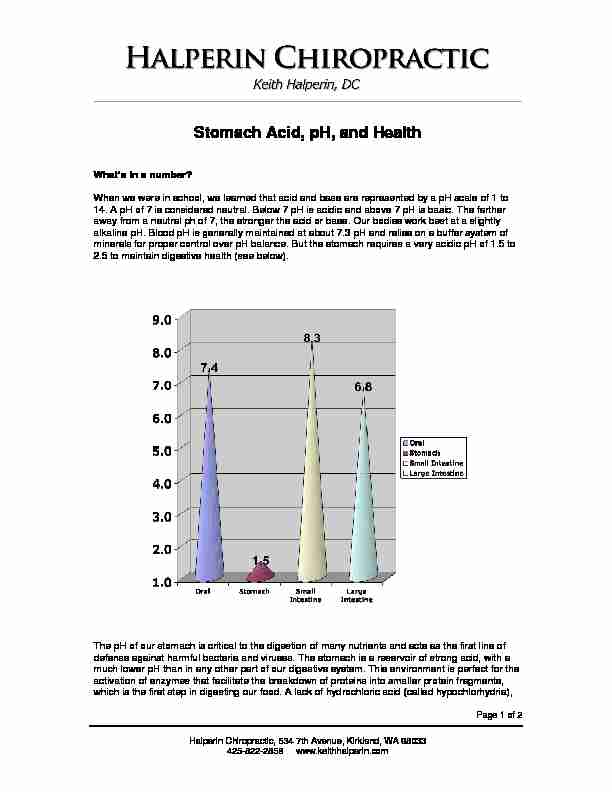
When we were in school, we learned that acid and base are represented by a pH scale of 1 to14. A pH of 7 is considered neutral. Below 7 pH is acidic and above 7 pH is basic. The farther
away from a neutral ph of 7, the stronger the acid or base. Our bodies work best at a slightly alkaline pH. Blood pH is generally maintained at about 7.3 pH and relies on a buffer system of minerals for proper control over pH balance. But the stomach requires a very acidic pH of 1.5 to2.5 to maintain digestive health (see below).
The pH of our stomach is critical to the digestion of many nutrients and acts as the first line of defense against harmful bacteria and viruses. The stomach is a reservoir of strong acid, with a much lower pH than in any other part of our digestive system. This environment is perfect for the activation of enzymes that facilitate the breakdown of proteins into smaller protein fragments, which is the first step in digesting our food. A lack of hydrochloric acid (called hypochlorhydria), Halperin Chiropractic, 634 7th Avenue, Kirkland, WA 98033425-822-2858 www.keithhalperin.com Page 2 of 2
the acid which determines stomach pH, affects the digestion of iron, folate, B12, calcium and protein.75% of our immune system is within our gut walls. This is because most bacterial invaders enter
through the mouth and nose. The stomach is our first line of defense as it contains acid and enzymes that dissolve the protein coats of bacteria, thereby either killing them or leaving them vulnerable to our immune responses. Stomach acid cleaves the bonds of mineral complexes attached to proteins and other food components, allowing them to be absorbed in the small intestine. Consider the use of antacids that advertise the addition of calcium carbonate as a valuable source of calcium. As they produce an alkaline pH in the stomach and provide the least absorbable (and cheapest) type of calcium, calcium carbonate, little to none of that calcium becomes available to the body. H. pylori are bacteria associated with stomach ulcers. H. pylori can be detected both in stool tests and in some blood tests. Sometimes the first indicator is gastritis or irritable bowel syndrome. This infection has a strong association with the lack of strong stomach acid found with aging and the use of antacids and proton pump inhibitors (Tagamet). As the reservoir of stomach acid becomes more alkaline (normal being pH 1.5), bacterial overgrowth occurs, which may lead to more serious conditions such as stomach cancer. Luckily, when H. pylori is found inthe stomach, it can be eradicated with antibiotics, fermented kefir, allicin, the active ingredient in
garlic, mastic gum and an antibiotic honey called manuka. Here are two helpful tips: garlic has to be raw and manuka honey should be of a strong grade from New Zealand. Since stomach pH is higher when we age or at times of prolonged stress, antacid use and a host of other factors, the ability to absorb nutrients and minerals is greatly diminished. Let's explore the connection to vitamin B12. The stomach produces intrinsic factor from specific cells within the stomach wall. These cells are induced to produce intrinsic factor only when the pH is within its normal acidic pH range. Intrinsic factor also bonds to B12, making it available to the gut microflora that absorbs B12 from the intestine. Without intrinsic factor, we are unable to assimilate B12. As we age, these cells produce less intrinsic factor. It is no wonder that vitamin B12 is one of three most deficient nutrients in most Americans, especially the elderly. This crucial nutrient is a cofactor in hundreds of enzyme reactions, including those that prevent heart disease and mental decline associated with age. Let's look at the big picture. If the acidic environment of the stomach is not maintained at the proper pH, we are unable to absorb minerals, proteins, and vitamins necessary for all the metabolic functions of the body. We can't make enzymes that catalyze (speed up or facilitate) digestion. We can't detoxify chemicals that enter the liver. We can't fight off disease causing bacteria and viruses. Our bodies must work tirelessly to maintain an alkaline blood and cell environment. Osteoporosis, for example, evolves partly due to a lack of available minerals to alkalinize the body. The minerals are leached from the bone, weakening the very structure of our bodies. As a result, our digestion and physiology suffer from unrelieved inflammation, the cause of all disease. When I use kinesiology to muscle-test a patient or discover nutrient depletions and changes in blood and urine tests that indicate inflammation and disease, I always make it a point to replenish both the enzymes of digestion, minerals, vitamins and of course the HCl (hydrochloric acid) needed for digestion until the body regains its balance and the ability to assimilate nutrients.quotesdbs_dbs2.pdfusesText_2