 Dalal Institute
Dalal Institute
Join the revolution by becoming a part of our community and get all of the member benefits like downloading any PDF document for your personal preview. Sign
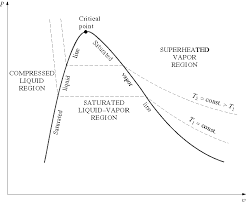 Thermodynamics Properties of Pure Substance
Thermodynamics Properties of Pure Substance
A substance is said to be superheated if the given temperature is greater than the saturation temperature for the given pressure. State 5 in Figure 2-3 (page 3)
 ENGINEERING THERMODYNAMICS
ENGINEERING THERMODYNAMICS
2 : Reversibe adiabatic process : p. 1. V. 1 γ = p. 2. V. 2 γ. V. V p p. 2. 1. 1. 2. 1. = F. HG. I. KJ γ. Page 160. FIRST LAW OF THERMODYNAMICS. 137 dharm. /M- ...
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
há 8 dias ... pdf. 2W. Kaplan 2003
 The two parts of the second law of thermodynamics
The two parts of the second law of thermodynamics
9 de jul. de 2018 That is the reason why in the processes (2) and (4) of the cycle the working agent is in isolation meaning that there is no exchange of heat.
 Thermodynamics Tables and Charts
Thermodynamics Tables and Charts
International Standard Formulation for the Thermodynamic Properties of 11
 UNIT – I – Thermodynamics-II – SCY1316
UNIT – I – Thermodynamics-II – SCY1316
UNIT – I – Thermodynamics-II – SCY1316. Page 2. 2. 1. INTRODUCTION. Need of Second Law of Thermodynamics. 1. First law states that “heat can be converted
 Fundamentals of Engineering Thermodynamics
Fundamentals of Engineering Thermodynamics
thermodynamics also deals with phenomena not included within the scope of mechanics ... 2 denote the inlet and exit respectively
 2 Thermodynamic Property Models
2 Thermodynamic Property Models
Aspen Physical Property System thermodynamic property models include classical thermodynamic property models such as activity coefficient models and equations
 Thermodynamics And An Introduction To Thermostatistics-Wiley
Thermodynamics And An Introduction To Thermostatistics-Wiley
Thermodynamics 1960. Bibliography p 485. Includes index. 1 Thermodynamics. I Callen Herbert B. 2 Statistical Mechanics. Thermodynamics II Title. III Title
 Chemical Engineering Thermodynamics II
Chemical Engineering Thermodynamics II
Chapter 2: Thermodynamic Property Relationships. 2.1. Type of Thermodynamic Properties. 2-1 4http://students.aiche.org/pdfs/thermodynamics.pdf 11/27/04.
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
Feb 14 2010 2.4.2 Non-ideal thermal equations of state . ... These are lecture notes for AME 20231
 ENGINEERING THERMODYNAMICS
ENGINEERING THERMODYNAMICS
Zeroth Law of Thermodynamics 23. 2.15. The Thermometer and Thermometric Property ... 24. 2.15.1. Introduction ... 24. 2.15.2. Measurement of temperature.
 Basic Concepts of Thermodynamics Thermodynamics and Energy
Basic Concepts of Thermodynamics Thermodynamics and Energy
velocity (m/s2) pressure (Pa = kg/m.s2). There are two unit systems currently available SI (International System) and USCS (United.
 The Second Law of Thermodynamics
The Second Law of Thermodynamics
2: Steam power plant is a heat engine. Thermal efficiency: is the fraction of the heat input that is converted to the net work output (efficiency = benefit /
 PDF First Law of Thermodynamics Control Volumes
PDF First Law of Thermodynamics Control Volumes
2: Energy content of CV can be changed by mass flow in/out and heat and work interactions. Work flow: is the energy that is required to push fluid into or out
 Introduction to chemical engineering thermodynamics
Introduction to chemical engineering thermodynamics
%20Hendrick%20Van%20Ness
 1 General Chemistry II Jasperse Entropy Spontaneity
1 General Chemistry II Jasperse Entropy Spontaneity
http://web.mnstate.edu/jasperse/Chem210/Extra%20Practice%20Sets%20Chem%20210/Test3ch14-Thermo-Practice.pdf
 Chapter 3 Thermodynamic Properties
Chapter 3 Thermodynamic Properties
48. Page 2. 3-2. Pressure can be expressed as a function of temperature and specific volume
 Thermodynamic Properties and calculation
Thermodynamic Properties and calculation
BASIC CONCEPTS-2. ? PV diagram. ? Virial Equations of State. PV = a + bP + cP2 + ... ?. ? Ideal gas: Z=1 or PV = RT.
 Lecture 5: Thermodynamics - Scholars at Harvard
Lecture 5: Thermodynamics - Scholars at Harvard
Lecture5: Thermodynamics 1 Introduction Thermodynamicsis thestudyof heat andtemperature Onethingthat makes thermodynamicshard(andgenerallyunpopular) is all the damnvariables Everything is relatedandit's oftentoughtokeepstraight whatisanindependentandwhat is adependent variable
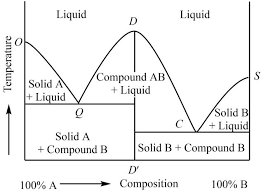
 Thermodynamics – II - Dalal Institute
Thermodynamics – II - Dalal Institute
Thermodynamics – II Clausius-Clapeyron Equation The Clausius-Clapeyron equation was initially proposed by a German physics Rudolf Clausius in 1834 and then further developed by French physicist Benoît Clapeyron in 1850 This equation is extremely
 Thermodynamics PDF: Definitions Basics Statements Laws and - BYJ
Thermodynamics PDF: Definitions Basics Statements Laws and - BYJ
ME 312– Thermodynamics II (Required) Catalog Description: ME 312 (3 0 3) continuation of ME 311 including studies of irreversibility and combustion Thermodynamic principles are applied to the analysis of power generation refrigeration and air-conditioning systems
 THERMODYNAMICS: COURSE INTRODUCTION
THERMODYNAMICS: COURSE INTRODUCTION
The thermodynamic state of a system is defined by specifying a setof measurable properties sufficient so that all remainingproperties are determined Examples of properties: pressuretemperature density internal energy enthalpy and entropy
What are the laws of thermodynamics?
Laws of thermodynamics gives a clear insight about energy, entropy, and thermal equilibrium of any system. The first law of thermodynamics, which is also known as the Law of Conservation of Energy, states that energy can neither be created nor be destroyed, it can only be transferred from one form to another.
What are the different types of thermodynamics?
Thermodynamic systems can be classified as follows: Open: Those systems that exchange matter and energy with the outside. Closed: Are those that exchange only energy with the outside. Isolated: Those that do not exchange neither energy nor matter.
What is the zeroth law of thermodynamics?
The Zeroth Law of thermodynamics states that there is an energy form called heat, which has the tendency to spread through a system, and a variable called temperature that measures this tendency: heat flows from the regions of high temperature to the regions of low temperature only. What is entropy law?
What are the applications of thermodynamics?
The understanding of thermodynamic phenomena is very useful in the fields of engineering, architecture, chemistry and biology. Especially, where enormous amounts of energy are needed to start up various machines. Also, the laws of thermodynamics help a lot in some disciplines such as genetics.