|
4 Spectroscopie Infra-rouge
4 1 Introduction Afin qu'un mode vibrationnel dans une molécule soit actif dans l'infrarouge il doit être associé à une modification du moment dipolaire permanent - Moment dipolaire d’une molécule c A > c B -q +q m Dipôle électrique : m = q d unité : C m ou Debye avec q = d×e 1 D = 334 10-30 C m |
|
Analyse spectrale Spectres IR
La spectroscopie infrarouge : un moyen de déterminer les groupes caractéristiques d’une molécule https://youtu be/U0Hu3-J0igE Animation par Ostralo net http://www ostralo net/3_animations/js/spectreIR/index htm Les corrigés sont rédigés par les professeurs de l’association Labolycée |
|
Interpretation of Infrared Spectra A Practical Approach
R A Meyers (Ed ) Copyright Ó John Wiley Sons Ltd The qualitative aspects of infrared spectroscopy are one of the most powerful attributes of this diverse and versatile analytical technique Over the years much has been published in terms of the fundamental absorption frequencies (also known as group frequencies) which are the key to unlocking t |
|
Résumé de cours : Spectres Infrarouge
Présentation d spectre IR En ordonnée la transmittance en qui représente le pourcentage de lumière ayant traversé l’échantillon En abscisse le nombre d’onde (l’inverse de la longueur d’onde) en cm-1 Il existe deux zones principales dans un spectre IR : |
|
Comprendre la spectroscopie infrarouge : principes et mise en
La mise en œuvre de l'interaction d’un rayonnement infrarouge avec un échantillon puis la détection et l'analyse spectrale (par transmission ou par réflexion) de ce rayonnement après qu'il ait interagi avec la matière est l'objet de la spectroscopie infrarouge |
Quel est le principe d'une spectroscopie IR ?
En conséquence, le principe d’une spectroscopie IR est d’envoyer des radiations IR sur un échantillon à tester. Certaines longueurs d’onde sont alors absorbées par les liaisons chimiques des molécules se trouvant dans l’échantillon. On génère alors un spectre IR, qui permet de déterminer ces liaisons chimiques.
Comment obtenir le spectre IR d'une molécule étudiée ?
Dans ce der-nier cas, le spectre IR de la molé-cule étudiée sera obtenu après soustraction du spectre IR du solvant utilisé. La qualité du spectre IR sera conditionnée par le choix des conditions expérimentales (trajet optique, concentration de la solution, nature des fenêtres de la cellule).
Comment interpréter un spectre IR ?
L’interprétation d’un spectre IR n’est pas chose facile, en particulier pour des molécules complexes. Voici quelques exemples simples, faisant apparaître la correspondance entre les bandes d’absorption et les liaisons chimiques :
Qu'est-ce que le spectre IR ?
Présentation d spectre IR En ordonnée, la transmittance en %, qui représente le pourcentage de lumière ayant traversé l’échantillon. Une seconde zone à droite correspondant à un nombre d’onde inférieur à 1400 cm-1, appelée « empreinte digitale » que nous ne pourrons pas analyser à cause de sa complexité. 3. Les groupes fonctionnels
Encyclopedia of Analytical Chemistry
R.A. Meyers (Ed.) Copyright Ó John Wiley & Sons Ltd The qualitative aspects of infrared spectroscopy are one of the most powerful attributes of this diverse and versatile analytical technique. Over the years, much has been published in terms of the fundamental absorption frequencies (also known as group frequencies) which are the key to unlocking t
2 THE ORIGINS OF THE INFRARED SPECTRUM
In the most basic terms, the infrared spectrum is formed as a consequence of the absorption of electromagnetic radiation at frequencies that correlate to the vibration of specific sets of chemical bonds from within a molecule. First, it is important to reflect on the distribution of energy possessed by a molecule at any given moment, defined as the
Etotal D Eelectronic C Evibrational C Erotational C Etranslational
.1/ The translational energy relates to the displacement of molecules in space as a function of the normal thermal motions of matter. Rotational energy, which gives rise to its own form of spectroscopy, is observed as the tumbling motion of a molecule, which is the result of the absorption of energy within the microwave region. The vibrational ener
E D hn
frequency=energy .2/ The fundamental requirement for infrared activity, lead-ing to absorption of infrared radiation, is that there must be a net change in dipole moment during the vibration for the molecule or the functional group under study. Another important form of vibrational spectroscopy is Raman spectroscopy, which is complementary
3 SPECTRAL INTERPRETATION BY APPLICATION OF VIBRATIONAL GROUP FREQUENCIES
This section includes tabulated data relative to the most significant group frequencies for the most common functional groups and structural components found in organic compounds. Brief reference is also made to simple inorganic compounds, in the form of simple ionic species. More detailed listings can be found in published literature, and the read
Methyl (−CH3)
Methyl C H asym./sym. stretch Methyl C H asym./sym. bend gem-Dimethyl or ‘‘iso’’- (doublet) Trimethyl or ‘‘tert-butyl’’ (multiplet) analyticalscience.wiley.com
Methyne ( CH )
− Methyne C H stretch Methyne C H bend Skeletal C C vibrations Special methyl ( analyticalscience.wiley.com
3.2 Simple Functional Groups
Obviously, there is a potentially broad number of molecular fragments that can be considered to be functional groups attached to an organic structure or backbone. This section features the most simple and most common of the functional groups, C X, i.e. the halogens (X D F, Cl, Br and I), hydroxy (X D OH), oxy or ether (X D OR, where R D alkyl), and
3.2.1 Halogenated Compounds
See Table 5. In principle, the interpretation of the spectra of molecules containing one or more halogens would seem to be straightforward. The functionality is simple, with just a single atom linked to carbon to form the group. With the polar nature of this group, one would expect the spectral contribution to be distinctive. In reality, this is no
3.2.2 Hydroxy and Ether Compounds
See Table 6 for alcohols and hydroxy compounds. The hydroxy function is probably one of the most dominant and characteristic of all of the infrared group frequencies. In most chemical environments, the hydroxy group does not exist in isolation, and a high degree of association is experienced as a result of extensive hydrogen bonding with other hydr
3.3 The Carbonyl Group
Carbonyl compounds are not only chemically important, but are also important in the interpretation of infrared spectra. The CDO absorption is almost always one of the most characteristic in the entire spectrum, and it is also most likely to be the most intense spectral feature. Table 9 provides an example listing of some of the common carbonyl freq
3.4 Other Functional Groups Associated with Heteroatoms
Potentially there are very large numbers of different organic-based compounds that are associated with one or more heteroatoms. These are in addition to the simple halogen- and amino-based compounds that have already been covered. A detailed discussion of such compounds is beyond the scope of this article. A few illustrative examples are included h
Nitrogen-oxy compounds
Aliphatic nitro compounds Aromatic nitro compounds Organic nitrates analyticalscience.wiley.com
4 THE PRACTICAL SITUATION – OBTAINING THE SPECTRUM AND INTERPRETING THE RESULTS
Up to this point, the fundamentals of interpretation have been discussed from the most basic concepts of infrared absorption by a molecular species and the impact of chemical functionality on the resultant spectrum. In many ways, this discussion has treated the molecule as a more or less isolated species, with no consideration of the physical state
4.1 Sample History
As noted, we are seldom dealing with a true unknown, and typical situations include the following: sample of fiber taken or extracted from a particular environment; contaminant removed from a material; suspicious liquid found leaking out of some drums; residue extracted from a surface; residual liquid or solid remaining after storage or treatment o
4.2 Physical Characteristics of the Sample
From considerations based on the discussions in the section above, a good working knowledge of the sample allows one to determine basic information about the sample. This information can help in the determination of the best method of sampling. It can also help to indicate if any special treatments to the sample are necessary before proceeding, suc
4.3 The Chemistry of the Sample
First, there are issues relating to solubility. The old adage, like dissolves like, is very appropriate. Nonpolar materials such as hydrocarbons tend to be more soluble in nonpolar solvents, such as hydrocarbons or chlorinated solvents, whereas polar materials favor solvents such as methanol and acetone, and in extreme cases their solubility may be
4.4 The Infrared Sampling Method
The final appearance of the infrared spectrum is very important in the interpretation process. Where possible, it always helps to be able to study the sample as it occurs naturally, without any form of physical modification. This eliminates the possibility of any interactions, or even chemical modifications. A common method for handling solid sampl
5 AN OVERVIEW TO INFRARED SPECTRAL INTERPRETATION – SOME SIMPLE RULES AND GUIDELINES
This final section is a review of the ideas presented so far as to how to go about the interpretation of an infrared spectrum. With it, there is the implied understanding that the infrared spectrum is only one source of information, and additional information is most likely required for a satisfactory analysis of the spectrum. The following are a s
Wavenumber (cm-1)
Figure 11 ATR spectrum of a commercial solvent mixture containing methanol, dichloromethane, toluene and acetone. Copyright Coates Consulting. and toluene. However, the use of separation techniques, such as water-based solvent extraction, and partial distillation can help in determining the identity of the components. Finally, in terms of using the
5.1.1 Step 1: Overall Spectrum Appearance
Is the spectrum simple? Are there only a few main characteristic absorption bands, say less than five? If the spectrum is simple, as defined, then the compound may be a low-molecular-weight organic or inorganic compound, such as a simple salt of a common molecular ion (carbonate, sulfate, nitrate, ammonium, etc.) or a covalent species (chloroform,
Infrared Spectroscopy (Volume 12)
Quantitative Analysis, Infrared Theory of Infrared Spectroscopy analyticalscience.wiley.com

Spectroscopie infrarouge (IR) ✅ Méthode Physique Chimie

Introduction to IR Spectroscopy: How to Read an Infrared Spectroscopy Graph

Interpréter un spectre Infrarouge IR
|
Fiche professeur Lanalyse spectrale : spectroscopies IR et RMN
théoriques ainsi que des exemples de spectres à analyser. Plan du document : Relier un spectre RMN simple à une molécule organique donnée. |
|
Analyse spectrale Spectres IR
La spectroscopie infrarouge : un moyen de déterminer les groupes caractéristiques d'une molécule Exploiter un spectre IR pour déterminer des groupes. |
|
Analyse spectrale Spectres IR
La spectroscopie infrarouge : un moyen de déterminer les groupes caractéristiques d'une molécule Exploiter un spectre IR pour déterminer des groupes. |
|
Comment déterminer la structure des molécules organiques ?
À quoi ressemble un spectre RMN du proton ? Comment faire pour exploiter cette information ... Méthode pour interpréter des spectres RMN de produits. |
|
II- SPECTROSCOPIE INFRAROUGE
INTERPRÉTATION DES SPECTRES. COMMENT FAIRE L'ANALYSE D'UN SPECTRE INFRAROUGE: Il est utile de savoir sur quoi se concentrer lorsqu'on doit faire l'analyse |
|
Spectroscopie infrarouge
Que voit-on sur un spectre IR ? Page 7. ? l'harmonique n'est pas exactement 2 fois la fondamentale : anharmonicité ! |
|
Spectroscopie infrarouge et analyse minéralogique quantitative des
15-Dec-1993 Un spectre infrarouge permet donc d'établir une correspondance entre les fréquences des bandes d'absorption et les paramètres. |
|
Contrôle des Connaissances de Spectroscopie Durée 1 h
06-Nov-2003 Attribuer les spectres IR et RMN 1H suivants aux composés ... comment la réalisation d'un spectre IR et d'un spectre RMN 1H sur le. |
|
Spectroscopic and rheological characterization of sodium alginate
L'analyse des alginates par spectroscopie infrarouge FT-IR ATR montre que les spectres de. D. polypodioides et D. ligulatus présentent une forte similarité |
|
Identification et dosage par spectrométrie infrarouge à transformée
Les spectres sont enregistrés entre 4000 et 600 cm-1 avec une accumulation de 32 spectres et une résolution de 2 cm-1. Spectre infrarouge de l'échantillon tel |
|
La spectroscopie infrarouge
bin/direct_frame_top cgi (très utile pour les spectres IR, les spectres RMN sont d'analyser les spectres pour en extraire les informations nécessaires à la Comment reconnaître des atomes d'hydrogène magnétiquement équivalents en |
|
Comprendre la spectroscopie infrarouge : principes et - Photoniques
du spectre électromagnétique La partie infrarouge du rayonnement électromagnétique est partagée en trois domaines : le proche infrarouge (le plus |
|
Spectroscopie infrarouge - Chimie en PCSI
III L'ALLURE DU SPECTRE IR 6 IV ORIGINES DIFFERENTES REGIONS DU SPECTRE INFRAROUGE 10 2 VI Interprétation d'un spectre infrarouge 1 |
|
Résumé de cours : Spectres Infrarouge
Il existe deux zones principales dans un spectre IR : appelée « empreinte digitale » que nous ne pourrons pas analyser à cause de sa complexité 3 |
|
Spectrométrie IR Principe - CNAM main page
Principe La spectrométrie infrarouge est l'un des outils les plus utilisés Principe Un spectre infrarouge est traditionnellement présenté en à analyser |
|
Application de la spectroscopie infrarouge à létude de lorganisation
de réaliser des spectres IR en transmission sur des échantillons en film mince (1, 3 mg) maintenu in l'étude des vibrations des molécules d'eau, l'interprétation des spectres infrarouges comment cet effet est visible sur certains spectres |
|
UTILISATION DE LA SPECTROSCOPIE IR - Mediachimie
Objectif Être capable de comprendre et analyser un spectre I R fiques pour analyser, identifier et caractériser les espèces et comment on l'utilise Document |
|
Comment déterminer la structure des molécules - Mediachimie
Comment faire pour exploiter cette information Exemples d 'interprétation de spectres IR et RMN Méthode pour interpréter des spectres RMN de produits |
|
Comment déterminer la structure des molécules - Mediachimie
[PDF] Comment déterminer la structure des molécules Mediachimie mediachimie sites RMN molecules organiques pdf |
|
spectroscopies IR et RMN - Phychim
[PDF] spectroscopies IR et RMN Phychim phychim ac versailles Documents formation spectroscopies pdf |
|
TP Analyse spectrale
[PDF] TP Analyse spectrale sciences edu Lycee louis payen reunion pdf |
|
Analyse spectrale Spectres IR - Labolycée
[PDF] Analyse spectrale Spectres IR Labolycéelabolycee Chimie Thema Chimie IR pdf |
|
Spectroscopie infrarouge (IR)
[PDF] Spectroscopie infrarouge (IR) lgtbellevue chapitre fonctions chimiques et spectres ir |
|
Spectroscopie infrarouge - ESPCI
[PDF] Spectroscopie infrarouge ESPCI cours espci site php?id= fileid= |
|
COURS DE SPECTROSCOPIE INFRAROUGE
[PDF] COURS DE SPECTROSCOPIE INFRAROUGE univ bouira dz Dr%MOUNI%Lotfi Chimie? |
|
Chapitre 4 : analyse spectrale
[PDF] Chapitre analyse spectralethierry col free restreint exovideo ch analyse spectrale pdf |
|
interpretation du spectre - e-Learn Université Ouargla
[PDF] interpretation du spectre e Learn Université Ouargla elearn univ ouargla dz interpretation%des%spectres%IR interpretationIR pdf ? |
|
SPECTROSCOPIES IR ET RMN : UNE AIDE À LA
Le spectre IR de l 'eau, enregistré avec un spectromètre basse résolution, fait Comment savoir si deux noyaux sont chimiquement équivalents ? souvent difficiles à interpréter, leur allure est imprévisible et leur étude détaillée n 'est pas à |
- comment analyser un spectre infrarouge
- analyse spectre rmn
- interprétation des spectres infrarouge pdf
- spectre infrarouge principe
- spectre infrarouge exercices corrigés
- spectre rmn exercice
- comment exploiter un spectre infrarouge
- spectroscopie rmn cours pdf

Spectre DEPT
Source:https://e.educlever.com/img/4/1/2/3/412384.jpg
Spectres infrarouge - Maxicours
Source:https://www.f-legrand.fr/scidoc/figures/numerique/tfd/analyse/figG.png

Analyse spectrale
Source:https://www.news-medical.net/image.axd?picture\u003d2019%2F4%2FBy_chromatos.jpg
Présentation des résultats de GC-MS dans une publication
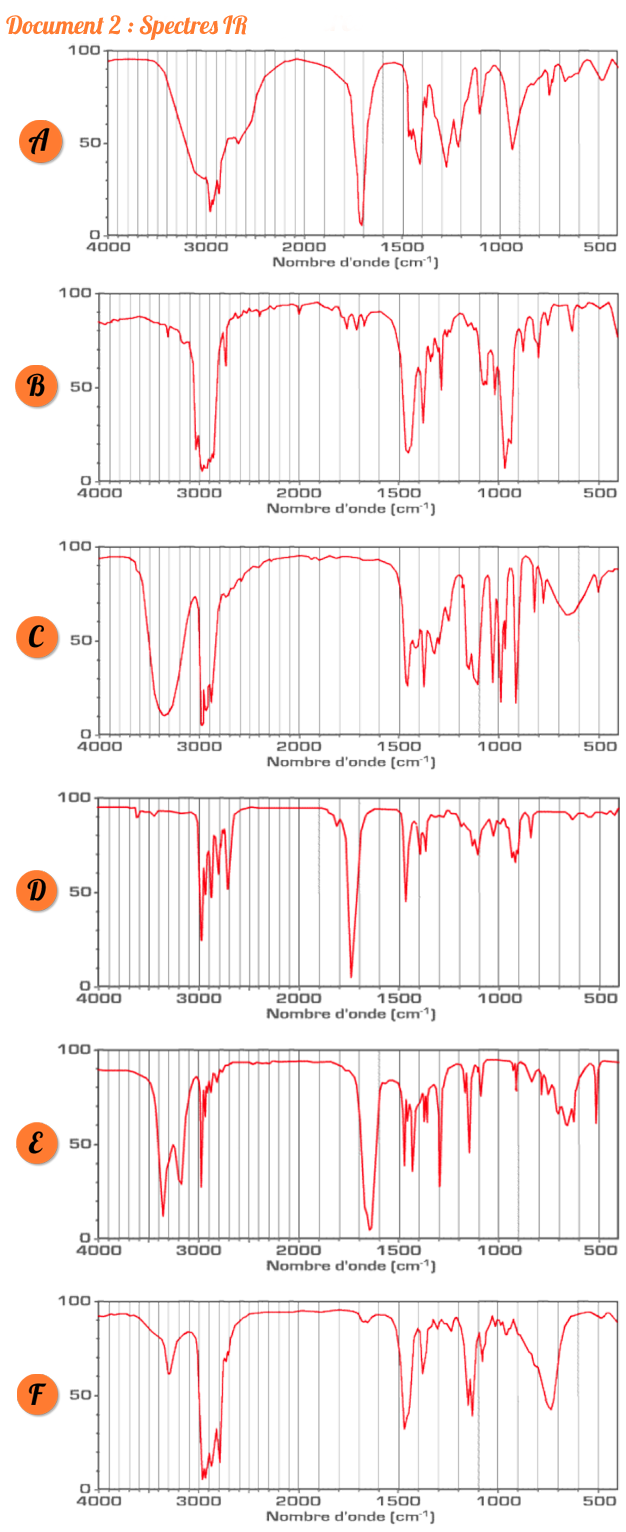
Source:http://lesbonsprofs.com/ressources/1-spectreIR%20copie.png
Spectroscopie Infrarouge Cours Et Exercices Pdf
Source: lwilsendithe's Ownd

Spectres infrarouge - Maxicours
Source:https://forums.futura-sciences.com/attachments/chimie/95820d1260525798-analyse-spectre-rmn-controle-continu-chimie-orga-p15-2a.pdf-adobe-reader.jpg
comment interpreter une experience en svt
Bien se représenter l 'expérience Analyser les données - SVT monde
- interpréter un document svt
- comment analyser un document en svt
- comment analyser et interpreter en svt
- analyse document svt seconde
- fiche méthode svt
- fiche méthode démarche d'investigation svt
comment investir pdf
Vos premiers pas dans l 'investissement - CBC
- comment investir son argent pdf
- comment gérer son argent pour devenir riche pdf
- comment économiser de l'argent au quotidien pdf
- investir quand on est étudiant
- comment investir son argent quand on est jeune pdf
- investir quand on est étudiant pdf
- comment economiser avec un petit salaire pdf
- comment investir son argent et faire du profit
comment investir son argent quand on est jeune pdf
investir : par où commencer - TesAffairescom
- comment économiser de l'argent au quotidien pdf
- comment gérer son argent pour devenir riche pdf
- comment economiser avec un petit salaire pdf
- comment investir pdf
- investir quand on est étudiant pdf
- comment investir son argent et faire du profit
comment isoler un toit par l'intérieur
ISOLER LA TOITURE INCLINÉE
- isolation toiture inclinée intérieur
- comment bien isoler son grenier
- toiture inclinée pdf
- isolation toiture par l'intérieur sans sous toiture
- toiture inclinée en béton
- prime isolation toiture
- isoler toiture par l'intérieur
- prix isolation toiture