 Chapter 20: Entropy and the Second Law of Thermodynamics
Chapter 20: Entropy and the Second Law of Thermodynamics
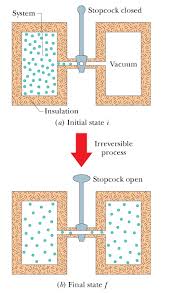
Heat does not transfer from the ice to the water (though this would not violate the law of energy conservation). AS > 0. Page 19. Sample Problem. Water is
 More problems on second law of thermodynamics
More problems on second law of thermodynamics
Understand the energy balance for a flow system and solve numericals on the applications on energy balance. Write the entropy balance equation.
 Untitled
Untitled
Thermodynamics problems: SOLUTIONS. Easy: Probability. Suppose you shuffle a dP. T=T₂. Page 15. A4 Using the differential form of the first law of ...
 1 Chapter 20 Entropy and second law of thermodynamics 1 Content
1 Chapter 20 Entropy and second law of thermodynamics 1 Content
S (extensive) F (extensive)
 Counterintuitive effect of gravity on the heat capacity of a metal
Counterintuitive effect of gravity on the heat capacity of a metal
18-Apr-2015 It is found that this solution violates the second law of thermodynamics ... problems-and-solutions/1967/1st_IPhO_1967.pdf. 15. Page 16. 2 Rudolf ...
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
31-Oct-2023 The objective of the course is to survey practical and theoretical problems in classical thermodynamics. The emphasis is on the axiomatic ...
 Solutions Manual for Thermodynamics and Chemistry
Solutions Manual for Thermodynamics and Chemistry
09-Jun-2020 The sign of S is positive as predicted by the second law for an irreversible process in an isolated system. 4.5 Refer to the apparatus shown ...
 Problem Solutions
Problem Solutions
The entropy of the oxide is 51.0 kJ/K per kilomole. According to the second law of thermodynamics the entropy of a closed system suffering any transformation
 The First Law of Thermodynamics: Closed Systems Heat Transfer
The First Law of Thermodynamics: Closed Systems Heat Transfer
Solution: Assume that i) the gas is a closed system ii) the moving boundary is only But from the second law point of view
 SECOND LAW OF THERMODYNAMICS (Numerical Problem
SECOND LAW OF THERMODYNAMICS (Numerical Problem
SECOND LAW OF. THERMODYNAMICS. (Numerical Problem Solution) e-content for B.Sc Physics (Honours). B.Sc Part-I. Paper-II. Dr. Ayan Mukherjee.
 ME 201
ME 201
Thermodynamics. Second Law Practice Problems. 1. Ideally which fluid can do more work: air at 600 psia and 600°F or steam at. 600 psia and 600°F. Solution:.
 Chapter 20: Entropy and the Second Law of Thermodynamics
Chapter 20: Entropy and the Second Law of Thermodynamics
Heat does not transfer from the ice to the water (though this would not violate the law of energy conservation). AS > 0. Page 19. Sample Problem. Water is
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
14-Feb-2010 These notes emphasize problem-solving and rigorous development of the ... 1850: Rudolf Julius Emanuel Clausius formalizes the second law of ...
 The First Law of Thermodynamics: Closed Systems Heat Transfer
The First Law of Thermodynamics: Closed Systems Heat Transfer
Solution: The energy content of the oven is increased during this process. a) The energy transfer to the oven is not caused by a temperature difference between
 Second Law and Entropy
Second Law and Entropy
Second law of thermodynamics - State the second law of thermodynamics as it Entropy - Solve problems involving the following concepts: (a) efficiency.
 Thermodynamic Properties and calculation
Thermodynamic Properties and calculation
First Law of Thermodynamic: ?Combining the first and second laws in reversible process ... other hand the answers to parts (a) and (b) show.
 Solution of Quiz 1: First Law of Thermodynamics
Solution of Quiz 1: First Law of Thermodynamics
12-Sept-2012 (d) The atom will undergo centre of mass motion with energy E3 ? E2. (e) The gaseous atoms collide with one another transferring the residual ...
 Problem Solutions
Problem Solutions
According to the second law of thermodynamics the entropy of a closed system suffering any transformation can not diminish.
 1 Solutions to sample quiz problems and assigned problems
1 Solutions to sample quiz problems and assigned problems
Quiz Problem 5. Use the Master equation to prove the second law of thermodynamics i.e. in a closed system. dS/dt ? 0. Solution. see lecture notes page 10.
 Second Law Problems - Michigan State University
Second Law Problems - Michigan State University
Thermodynamics Second Law Practice Problems Ideally which fluid can do more work: air at 600 psia and 600°F or steam at600 psia and 600°F Solution: The maximum work a substance can do is given by its availablity We willassume that we have a closed system so that÷
 Thermodynamics: The Three Laws of Thermodynamics - Equations
Thermodynamics: The Three Laws of Thermodynamics - Equations
The second law of thermodynamics asserts that processes occur in a certain direction and that the energy has quality as well as quantity The first law places no restriction on the direction of a process and satisfying the first law does not guarantee that the process will occur
 Chapter 7 THE SECOND LAW OF THERMODYNAMICS
Chapter 7 THE SECOND LAW OF THERMODYNAMICS
Second Law of Thermodynamics and Thermal Energy Reservoirs 7-1C Water is not a fuel; thus the claim is false 7-2C Transferring 5 kWh of heat to an electric resistance wire and producing 5 kWh of electricity 7-3C An electric resistance heater which consumes 5 kWh of electricity and supplies 6 kWh of heat to a room
 Chapter 5 The Second Law of Thermodynamics - Saylor Academy
Chapter 5 The Second Law of Thermodynamics - Saylor Academy
The second law of thermodynamics states that processes occur in a certain direction not in just any direction Physical processes in nature can proceed toward equilibrium spontaneously: Water flows down a waterfall Gases expand from a high pressure to a low pressure Heat flows from a high temperature to a low temperature
 Searches related to second law of thermodynamics problems and solutions pdf filetype:pdf
Searches related to second law of thermodynamics problems and solutions pdf filetype:pdf
2 Second law of thermodynamics If a closed system is in a configuration that is not the equilibrium configuration the most probable consequence will be that the entropy of the system will increase monotonically If an irreversible process occurs in a closed system the entropy of the system always increases; it never decreases
What are the first three laws of thermodynamics?
- There three laws are: The first law of thermodynamics is the law of the conservation of energy; it states that energy cannot be created nor destroyed. An example is when the chlorophyll absorbs light and transforms it into chemical energy.
How many laws of thermodynamics are there?
- Traditionally, thermodynamics has recognized three fundamental laws, simply named by an ordinal identification, the first law, the second law, and the third law. [1] [2] [3] A more fundamental statement was later labelled as the zeroth law, after the first three laws had been established.
What does first law of thermodynamics mean?
- The first law of thermodynamics, also known as the law of conservation of energy states that energy can neither be created nor destroyed, but it can be changed from one form to another. According to this law, some heat given to the system is used to change the internal energy while the rest is used in doing work by the system.
ME 201 Thermodynamics
1ME 201
Thermodynamics
Second Law Practice Problems
1.Ideally, which fluid can do more work: air at 600 psia and 600°F or steam at
600 psia and 600°F
Solution:
The maximum work a substance can do is given by its availablity. We will assume that we have a closed system so that y = u-u - T(s-s) ooo We take the dead state to be at STP or 25°C and 100 kPa or 76.4°F and 14.7 psia.Then using the appropriate table we have
yair m = 183.30-91.53 - (537)0.7649-0.5995-0.06855ln600 14.7 = 139.48 Btu/lb éûúae
and()ysteam m = 1184.5-44.09 - (537)1.5320-0.08215 = 361 .84 Btu/lbSo the steam can do more work
2.A heat pump provides 30,000 Btu/hr to maintain a dwelling at 68°F on a day
when the outside temperature is 35°F. The power input to the pump is 1 hp. If electricity costs 8 cents per kilowatt-hour, compare the actual operating cost per day with the minimum theoretical operating cost per day.ME 201 Thermodynamics
2Solution:
We sketch our device interactions
The cost is given by
Cost = (0.08)W
netFor the actual cost we have
(Cost) = (0.08)(1)(0.7457 kW/hp)(24 hr/day) = $1.43 act To calculate the minimum cost we will allow the heat pump to operate as a Carnot cycle, so thatCOP = 1
TT = 1
495528 = 16CarnotL
H 11--Then the minimum possible power input is
()&&W = QCOP = 30,000
16 = 1875 Btu/hr
= 0.5495 kW netminHCarnot
High Temperature Heat Reservoir
at THLow Temperature Heat Reservoir
at TLHeat Pump
QL QH WnetDwelling
Outside
ME 201 Thermodynamics
3 and the minimum cost is (Cost) = (0.08)(0.5495)(24 hr/day) = $1.06 min3.A cylinder/piston system contains water at 200 kPa, 200°C with a volume of 20
liters. The piston is moved slowly, compressing the water to a pressure of 800 kPa. The process is polytropic with a polytropic exponent of 1. Assuming that the room temperature is 20°C, show that this process does not violate the second law.Solution:
To determine if this violate the 2nd law we will want to calculate the entropy change of the universe and compare it to zero. We have ()DS = m(s-s) + -QT universe21sys
surrWe now work this as a first law problem
Working Fluid: Water(compressible)
System: Closed System
Process: Polytropic with n=1.0
State 1
State 2
T1 = 200°CT2 = 214.7°C
P1 = 200 kPaP2 = 800 kPa
u1 = 2654.4 kJ/kgu2 = 2655.5kJ/kg
V1 = 0.020 m3V2 = 0.005 m3
v1 = 1.0803 m3/kgv2 = 0.2703 m3/kg
s1 = 7.5066 kJ/(kg K)s2 = 6.8811 kJ/(kg K)
phase: sup.vap.phase: sup.vap. italicized values from tables, bold values are calculatedInitial State: Fixed
Final State: Unknown
W sh = 0Q = ????
W bnd = ????We begin by calculating the mass
m = V v = 0.0201.0803 = 0.0185 kg 1
1ME 201 Thermodynamics
4 To fix the final state we use the polytropic relationshipV = PV
P = (200)(0.020)
800= 0.005 m 211n
21/n11/1
3éThe specific volume at state 2 is then
v = Vm = 0.0050.0185 = 0.2703 kg/m 223 which gives us superheated vapor. The boundary work can be shown to beW = PVlnV
V = (200)(0.020)ln0.005
0.020 = -5.55 kJ bnd1121×é
ûúae
We use the first law to determine the heat transfer Q = m(u-u) + W = (0.0185)(2655.5-2654.4)+(-5.55)= -5.53 kJ sys21 Then ()DS = -QT = -(-5.53)
293 = 0.0189 kJ/K surroundssys
surr and for the system ()DS = m(s-s) = (0.0185)(6.8811-7.5066) = -0.0129 kJ/K system21So that
()DS = -0.0129 + 0.0189 = 0.006 kJ/K universe Since this is greater than zero the second law is not violated.4.When a man returns to his well-sealed house on a summer day, he finds that
the house is at 32°C. He turns on the air conditioner which cools the entire house to 20°C in 15 minutes. If the COP of the heat pump system is 2.5, determine the power drawn by the heat pump. Assume the entire mass within the house is equivalent to 800 kg of air.ME 201 Thermodynamics
5Solution:
We begin by sketching our device interactions
By definition we have
COP = Q
W H net So if the required heat transfer can be determined the power can be determined. From a first law analysis on the house, we can write &Q = mu-u t = (800)209.06-217.67 (15)(60) = -7.65 kW 21and &&Q = -Q = 7.65 kW H
Then the power required is
&&W = QCOP = 7.652.5 = 3.06 kW netHHigh Temperature Heat Reservoir
at THLow Temperature Heat Reservoir
at TLHeat Pump
QL QH Wnet HouseAC System
ME 201 Thermodynamics
65.An innovative way of power generation involves the utilization of geothermal
energy, the energy of hot water that exists naturally underground (hot springs), as the heat source. If a supply of hot water at 140°C is discovered at a location where the environmental temperature is 20°C, determine the maximum thermal efficiency a geothermal plant built at that location can have. If the power output of the plant is to be 5 MW, what is the minimum mass flow rate of hot water needed?Solution:
We begin by sketching our device interactions
The maximum thermal efficiency will occur when the heat engine operates as aCarnot cycle,
hhthCarnotL H = = 1 - TT = 1 - (20+273)
(140+273) = 0.291 The minimum mass flow rate of hot water corresponds to the maximum thermal efficiency or .Q = W = 5000 = 17,208 kW HminnetCarnoth0291High Temperature Heat Reservoir
at THLow Temperature Heat Reservoir
at TLHeat Engine
QL QH WnetGeothermal Source
Environment
ME 201 Thermodynamics
7 Performing a first law analysis on the hot water stream we have ()Q = mh-h outin& For the minimum flow rate we will assume that the hot water is cooled down to the environment temperature, then ()()&.)m = Q cTT = -17,208 (20140 = 34.2 kg/s Poutin--419786.Air enters an adiabatic non-ideal nozzle at 9 m/s, 300 K, and 120 kPa and exits
at 100 m/s and 100 kPa. Determine the irreversibility and the reversible work on a per mass basis.Solution:
We first solve this as a first law problem
Working Fluid: Air(ideal gas)
System: Control Volume System
Process: Nozzle
State 1
State 2
T1 = 300KT2 = 295°C
P1 = 120 kPaP2 = 100 kPa
h1 = 300.19 kJ/kgh2 = 295.04 kJ/kg
f1 = 1.70203 kJ/(kg K)f2 = 1.68515 kJ/(kg K)
rv1 = 9 m/srv2 = 100 m/s italicized values from tables, bold values are calculatedInitial State: Fixed
Final State: ???
W sh = 0 Q = 0We use the first law to fix the final state
h + v2 = h + v2 1122 rrThen solving for h2
h = h + vv2 = 300.19+(9)(10)
= 295.04 kJ/kg 2112222-3 rr--()100 22which allows us to determine the temperature and f2. Then the reversible work is
ME 201 Thermodynamics
8 w = h-h - T--RlnP P = 300.19-295.04 - (298)1.70203-1.68515-0.287)ln120 100= 15.71 kJ/kg rev12HR121
2ff×é
ûúae
ûúae
Since the actual work is zero the irreversibility is i = w = 15.71 kJ/kg rev7.Determine if a tray of ice cubes could remain frozen when placed in a food
freezer having a COP of 9, operating in a room where the temperature is 32°C.Solution:
We begin by sketching our device interactions
High Temperature Heat Reservoir
at THLow Temperature Heat Reservoir
at TLRefrigerator
QL QH WnetSurroundings
Freezer Compartment
ME 201 Thermodynamics
9 Assuming that the refrigerator operates on the Carnot cycle, we haveCOP = COP = 1
T TCarnotH
L -1Solving for TL
T = T 1 COP = 305 1 9 = 274.5 K LH ++11 and since this is greater than 0°C the ice cubes will not remain frozen.8.Air is compressed in a closed system from a state where the pressure is 100 kPa
and the temperature is 27°C to a final state at 500 kPa and 177°C. Can this process occur adiabatically? If yes, determine the work per mass. If no, determine the direction of the heat transfer.Solution:
To determine if the process can occur, we must calculate ()DS = m(s-s) + -QT universe21sys
surr and compare it to zero. Since the process is adiabatic ()Ds = s-s = --RlnPP universe21212
1ff×ae
Going to the air tables we find()Ds = s-s = 2.11161-1.70203-0.287)ln500 100= - 0.523 kJ/kg universe21(ae Since this is less than zero, the process cannot be adiabatic. To make Dsuniverse greater than zero will require Qsys to be negative, so that the direction of heat transfer is out of the system.
9.The pressure of water is increased by the use of a pump from 14 to 40 psia. A
rise in the water temperature from 60°F to 60.2°F is observed. Determine the irreversibility, the second law efficiency, and the isentropic efficiency of the pump.ME 201 Thermodynamics
10Solution:
We first solve this as a first law problem
Working Fluid: Water (incompressible)
System: Control Volume System
Process: Pump
State 1
State 2s (ideal)State 2a (actual
T1 = 60°FT2s =T2a = 60.2°F
P1 = 14 psiaP2 = 40 psiaP2 = 40 psia
bold values are calculatedInitial State: Fixed
Final State: fixed
W sh = ???? Q = 0To calculate the irreversibility, we use
i = T(s-s) - q = (537)clnT T - 0 = (537)(1.0014)ln520.2 520= 0.2068 Btu/lb HR21 p,avg2 1 m ae ae To determine the second law efficiency we need both the actual work and the ideal work. Starting with the actual work we have w = h - h + q = c(T-T) + v(P-P) - 0 = (1.0014)(60-60.2) + (0.016035)(14-40)/(5.40395 psiaft/Btu) = -0.2774 Btu/lb act12 p,avg12avg12 3 m
The reversible work is given by
w = i + w = (0.2068) + (-0.2774) = -0.0706 Btu/lbrevact m which allows us to determine the second law efficiency as hIIrev act = w w = (-0.0706) (-0.2774) = 0.255ME 201 Thermodynamics
11To determine the isentropic efficiency, we must first calculate the ideal work.
Recognizing that in a isentropic process, the water will not change temperature, we can write w = v(P-P) = (0.016035)(14-40)/(5.40395 psiaft/Btu) = -0.0771 Btu/lb idealavg12 3 mThen our isentropic efficiency is
hsideal act = w w = (-0.0771) (-0.2774) = 0.278110. Carbon dioxide undergoes an isothermal reversible process from 250 kPa and
300°C to 500 kPa. Determine the heat transfer per mass by using the first law
and evaluating the boundary work from Pdvò . Compare this to the heat
transfer per mass calculated from the entropy change and the second law.Solution:
We first solve this as a first law problem
Working Fluid: CO2 (ideal gas)
System: Closed System
Process: Isothermal, Reversible
State 1
State 2
T1 = 300C = 573KT2 = T1 = 573K
P1 = 250 kPaP2 = 500 kPa
u1 = 369.23 kJ/kgu2 = 369.23 kJ/kg
f1 = 5.478 kJ/(kg K)f2 = 5.478 kJ/(kg K)
v1 = 0.433 m3/kgv2 = 0.2165 m3/kg
italicized values are from ideal gas relationshipsInitial State: Fixed
Final State: fixed
W sh = 0Q = ???
W bnd = ????ME 201 Thermodynamics
12We first go to the CO2 tables and get our properties. Our boundary work for an
ideal gas undergoing an isothermal process is w = RTlnv v = (0.1889)(573)ln0.2165 0.433 = -75.03 Btu/lb bnd2 1 m×ae
aeUsing the first law our heat transfer is
q = u-u+w = 369.23-369.23+(-75.036) = -75.036 Btu/lb 21bnd mFrom the second law we have
q = T(s-s) = T--RlnP P = (573)5.478-5.478-0.1889)ln500 250= -75.03 Btu/lb 121 1 212
1 m ff×é
ûúae
ûúae
So the two calculations for heat transfer agree.
quotesdbs_dbs5.pdfusesText_10[PDF] second order differential equation rlc circuit
[PDF] second order low pass filter
[PDF] second trimester bleeding differential diagnosis
[PDF] second year computer engineering syllabus pune university 2019 20
[PDF] secondary amine reaction with hcl
[PDF] secondary amine reaction with nano2 and hcl
[PDF] secondary amine reaction with water
[PDF] secondary colors of light
[PDF] secondary consumers are eaten by larger
[PDF] secondary consumers in the desert
[PDF] secondary consumers in the ocean
[PDF] secondary consumers in the rainforest
[PDF] secondary consumers in the savanna
[PDF] secondary consumers in the tundra
