 ME 201
ME 201
ME 201 Thermodynamics. 1. ME 201. Thermodynamics. Second Law Practice Problems. 1. Ideally which fluid can do more work: air at 600 psia and 600°F or steam at.
 Chapter 20: Entropy and the Second Law of Thermodynamics
Chapter 20: Entropy and the Second Law of Thermodynamics
Heat does not transfer from the ice to the water (though this would not violate the law of energy conservation). AS > 0. Page 19. Sample Problem. Water is
 More problems on second law of thermodynamics
More problems on second law of thermodynamics
Understand the energy balance for a flow system and solve numericals on the applications on energy balance. Write the entropy balance equation.
 Untitled
Untitled
Thermodynamics problems: SOLUTIONS. Easy: Probability. Suppose you shuffle a dP. T=T₂. Page 15. A4 Using the differential form of the first law of ...
 1 Chapter 20 Entropy and second law of thermodynamics 1 Content
1 Chapter 20 Entropy and second law of thermodynamics 1 Content
S (extensive) F (extensive)
 Counterintuitive effect of gravity on the heat capacity of a metal
Counterintuitive effect of gravity on the heat capacity of a metal
18-Apr-2015 It is found that this solution violates the second law of thermodynamics ... problems-and-solutions/1967/1st_IPhO_1967.pdf. 15. Page 16. 2 Rudolf ...
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
31-Oct-2023 The objective of the course is to survey practical and theoretical problems in classical thermodynamics. The emphasis is on the axiomatic ...
 Problem Solutions
Problem Solutions
The entropy of the oxide is 51.0 kJ/K per kilomole. According to the second law of thermodynamics the entropy of a closed system suffering any transformation
 The First Law of Thermodynamics: Closed Systems Heat Transfer
The First Law of Thermodynamics: Closed Systems Heat Transfer
Solution: Assume that i) the gas is a closed system ii) the moving boundary is only But from the second law point of view
 SECOND LAW OF THERMODYNAMICS (Numerical Problem
SECOND LAW OF THERMODYNAMICS (Numerical Problem
SECOND LAW OF. THERMODYNAMICS. (Numerical Problem Solution) e-content for B.Sc Physics (Honours). B.Sc Part-I. Paper-II. Dr. Ayan Mukherjee.
 ME 201
ME 201
Thermodynamics. Second Law Practice Problems. 1. Ideally which fluid can do more work: air at 600 psia and 600°F or steam at. 600 psia and 600°F. Solution:.
 Chapter 20: Entropy and the Second Law of Thermodynamics
Chapter 20: Entropy and the Second Law of Thermodynamics
Heat does not transfer from the ice to the water (though this would not violate the law of energy conservation). AS > 0. Page 19. Sample Problem. Water is
 LECTURE NOTES ON THERMODYNAMICS
LECTURE NOTES ON THERMODYNAMICS
14-Feb-2010 These notes emphasize problem-solving and rigorous development of the ... 1850: Rudolf Julius Emanuel Clausius formalizes the second law of ...
 The First Law of Thermodynamics: Closed Systems Heat Transfer
The First Law of Thermodynamics: Closed Systems Heat Transfer
Solution: The energy content of the oven is increased during this process. a) The energy transfer to the oven is not caused by a temperature difference between
 Second Law and Entropy
Second Law and Entropy
Second law of thermodynamics - State the second law of thermodynamics as it Entropy - Solve problems involving the following concepts: (a) efficiency.
 Thermodynamic Properties and calculation
Thermodynamic Properties and calculation
First Law of Thermodynamic: ?Combining the first and second laws in reversible process ... other hand the answers to parts (a) and (b) show.
 Solution of Quiz 1: First Law of Thermodynamics
Solution of Quiz 1: First Law of Thermodynamics
12-Sept-2012 (d) The atom will undergo centre of mass motion with energy E3 ? E2. (e) The gaseous atoms collide with one another transferring the residual ...
 Problem Solutions
Problem Solutions
According to the second law of thermodynamics the entropy of a closed system suffering any transformation can not diminish.
 1 Solutions to sample quiz problems and assigned problems
1 Solutions to sample quiz problems and assigned problems
Quiz Problem 5. Use the Master equation to prove the second law of thermodynamics i.e. in a closed system. dS/dt ? 0. Solution. see lecture notes page 10.
 Second Law Problems - Michigan State University
Second Law Problems - Michigan State University
Thermodynamics Second Law Practice Problems Ideally which fluid can do more work: air at 600 psia and 600°F or steam at600 psia and 600°F Solution: The maximum work a substance can do is given by its availablity We willassume that we have a closed system so that÷
 Thermodynamics: The Three Laws of Thermodynamics - Equations
Thermodynamics: The Three Laws of Thermodynamics - Equations
The second law of thermodynamics asserts that processes occur in a certain direction and that the energy has quality as well as quantity The first law places no restriction on the direction of a process and satisfying the first law does not guarantee that the process will occur
 Chapter 7 THE SECOND LAW OF THERMODYNAMICS
Chapter 7 THE SECOND LAW OF THERMODYNAMICS
Second Law of Thermodynamics and Thermal Energy Reservoirs 7-1C Water is not a fuel; thus the claim is false 7-2C Transferring 5 kWh of heat to an electric resistance wire and producing 5 kWh of electricity 7-3C An electric resistance heater which consumes 5 kWh of electricity and supplies 6 kWh of heat to a room
 Chapter 5 The Second Law of Thermodynamics - Saylor Academy
Chapter 5 The Second Law of Thermodynamics - Saylor Academy
The second law of thermodynamics states that processes occur in a certain direction not in just any direction Physical processes in nature can proceed toward equilibrium spontaneously: Water flows down a waterfall Gases expand from a high pressure to a low pressure Heat flows from a high temperature to a low temperature
 Searches related to second law of thermodynamics problems and solutions pdf filetype:pdf
Searches related to second law of thermodynamics problems and solutions pdf filetype:pdf
2 Second law of thermodynamics If a closed system is in a configuration that is not the equilibrium configuration the most probable consequence will be that the entropy of the system will increase monotonically If an irreversible process occurs in a closed system the entropy of the system always increases; it never decreases
What are the first three laws of thermodynamics?
- There three laws are: The first law of thermodynamics is the law of the conservation of energy; it states that energy cannot be created nor destroyed. An example is when the chlorophyll absorbs light and transforms it into chemical energy.
How many laws of thermodynamics are there?
- Traditionally, thermodynamics has recognized three fundamental laws, simply named by an ordinal identification, the first law, the second law, and the third law. [1] [2] [3] A more fundamental statement was later labelled as the zeroth law, after the first three laws had been established.
What does first law of thermodynamics mean?
- The first law of thermodynamics, also known as the law of conservation of energy states that energy can neither be created nor destroyed, but it can be changed from one form to another. According to this law, some heat given to the system is used to change the internal energy while the rest is used in doing work by the system.
Solutions Manual for
Thermodynamics and Chemistry
Second Edition
byHoward DeVoe
Associate Professor of Chemistry Emeritus
University of Maryland, College Park, Maryland
hdevoe@umd.eduCopyright 2020 by Howard DeVoe
This work is licensed under a Creative Commons Attribution 4.0 International License:Contents
Preface
31 Introduction
42 Systems and Their Properties
53 The First Law
84 The Second Law
165 Thermodynamic Potentials
196 The Third Law and Cryogenics
247 Pure Substances in Single Phases
268 Phase Transitions and Equilibria of Pure Substances
369 Mixtures
4110 Electrolyte Solutions
5511 Reactions and Other Chemical Processes
5812 Equilibrium Conditions in Multicomponent Systems
7713 The Phase Rule and Phase Diagrams
9414 Galvanic Cells
104Preface
This manual contains detailed solutions to the problems appearing at the end of each chapter of the textThermodynamics and Chemistry. Each problem printed in the text is reproduced in this manual, followed by a worked-out solution. If a figure or table accompanies a problem in the text, it is also reproduced here. Included within a solution may be an additional figure or table that does not appear in the text. All figures, tables, and footnotes in this manual are numbered consecutively (Figure 1, Figure 2, etc.) and so do not agree with the numbering in the text. In most cases of a numerical calculation involving physical quantities, the setup in this manual shows the values of given individual physical quantities expressed in SI base units and SI derived units, without prefixes. The result of the calculation is then expressed in SI base units and SI derived units appropriate to the physical quantity being evaluated. Since the factors needed to convert the units of the given quantities to the units of the calculated quantity all have numerical values of unity when this procedure is followed, the conversion factors are not shown. Of course, the solution given in this manual for any particular problem is probably not the only way the problem can be solved; other solutions may be equally valid. 4Chapter 1 Introduction
1.1 Consider the following equations for the pressure of a real gas. For each equation, find the dimensions of the constantsaandband express these dimensions in SI units. (a)The Dieterici equation:
pDRTe.an=VRT/ .V=n/bSolution:
Sincean=VRTis a power, it is dimensionless andahas the same dimensions as VRT=n. These dimensions are volumeenergy/amount2, expressed in m3Jmol2.bhas the same dimensions asV=n, which are volume/amount expressed in m3mol1. (b)The Redlich-Kwong equation:
pDRT .V=n/ban2 T1=2V.VCnb/
Solution:
The terman2=T1=2V.VCnb/has the same dimensions asp, soahas the same dimensions asT1=2V2pn2. The SI units are K1=2m6Pamol2.bhas the same dimensions asV=n, which are volume/amount expressed in m3mol1. 5Chapter 2 Systems and Their Properties
2.1 LetXrepresent the quantityV2with dimensions.length/6. Give a reason thatXis or is not an extensive property. Give a reason thatXis or is not an intensive property.Solution:
1 Xis not an intensive property because it is dependent on volume. 2.2 Calculate therelative uncertainty(the uncertainty divided by the value) for each of the mea- surement methods listed in Table 2.2 on page 38, using the typical values shown. For each of the five physical quantities listed, which measurement method has the smallest relative uncer- tainty?
Solution:
Mass: analytical balance ,0:1103g=100gD1106 micro balance,0:1106g=20103gD5106Volume:
pipet,0:02ml=10mLD2103 volumetric flask ,0:3103L=1LD3104Density:
pycnometer,2103gmL1=1gmL1D2103 magnetic float densimeter ,0:1103gmL1=1gmL1D1104Pressure:
manometer or barometer ,0:001Torr=760TorrD1106 diaphragm gauge,1Torr=100TorrD1102Temperature:
gas thermometer,0:001K=10KD1104 mercury thermometer,0:01K=300KD3105 platinum resistance thermometer ,0:0001K=300KD3107 optical pyrometer,0:03K=1300KD2105 The measurement of temperature with a platinum resistance thermometer has the least relative uncertainty, and the measurement of pressure with a diaphragm gauge has the greatest. For each physical quantity, the measurement method with smallest relative uncertainty is underlined in the preceding list. 2.3 Table 1 samples of varying amounts of helium maintained at a certain fixed temperatureT2in the gas bulb. 1 The molar volumeVmof each sample was evaluated from its pressure in the bulb at a reference temperature ofT1D7:1992K, corrected for gas nonideality with the known value of the second virial coefficient at that temperature.Use these data and Eq.
2.2.2 on page 34to evaluateT2and the second virial coefficient of he- lium at temperatureT2. (You can assume the third and higher virial coefficients are negligible.)
Solution:
With the third and higher virial coefficients set equal to zero, Eq. 2.2.2 becomes pV mDRT 1CB V m 1Ref. [
13 6Table 1
Helium at a fixed temperature
1=Vm/=102molm3.p2Vm=R/=K
1.02252.7106
1.32022.6994
1.58292.6898
1.90422.6781
2.45722.6580
2.81802.6447
3.41602.6228
3.60162.6162
4.13752.5965
4.61152.5790
5.17172.5586??
0 1 2 3 4 5 62:52:62:7
2.8 .1=V m/=102molm3 .p2Vm=R/=KFigure 1
According to this equation, a plot ofp2Vm=Rversus1=Vmshould be linear with an intercept at1=VmD0equal toT2and a slope equal toBT2. The plot is shown in Fig. 1 . A least-squares fit of the data to a first-order polynomial yields an intercept of2:7478K and a slope of3:659104Km3mol1. The temperature and second virial coefficient therefore have the
values T2D2:7478K
BD3:659104Km3mol1
2:7478KD 1:332104m3mol1
2.4 Discussthepropositionthat, toacertaindegreeofapproximation, alivingorganismisasteady- state system.Solution:
The organism can be treated as being in a steady state if we assume that its mass is constant 7 and if we neglect internal motion. Matter enters the organism in the form of food, water, and oxygen; waste matter and heat leave the system. 2.5 feasibility of measuring this mass change.Solution:
This mass change is much less than the uncertainty of a microbalance (Table 2.2 ), which does not even have the capacity to weigh one mole of KI - so it is hopeless to try to measure this mass change. 8Chapter 3 The First Law
3.1 Assume you have a metal spring that obeys Hooke"s law:FDc.ll0/, whereFis the force exerted on the spring of lengthl,l0is the length of the unstressed spring, andcis the spring constant. Find an expression for the work done on the spring when you reversibly compress it from lengthl0to a shorter lengthl0.Solution:
wDZ l0 l0FdlDcZ
l0 l0.ll0/dlD1
2 c.ll0/2l0 l 0D1 2 c.l0l0/2waterairFigure 2
3.2The apparatus shown in Fig.
2 consists of fixed amounts of water and air and an incompressible solid glass sphere (a marble), all enclosed in a rigid vessel resting on a lab bench. Assume the marble has an adiabatic outer layer so that its temperature cannot change, and that the walls of the vessel are also adiabatic. Initially the marble is suspended above the water. When released, it falls through the air into the water and comes to rest at the bottom of the vessel, causing the water and air (but not the marble) to become slightly warmer. The process is complete when the system returns to an equilibrium state. The system energy change during this process depends on the frame of For each of the following definitions of the system, give thesign(positive, negative, or zero) of both center-of-mass frame.Solution:
We can use Eq.
3.1.4 (a)The system is the marble.
Solution:
sysis negative, because in the lab frame the marble does work on the water (page 839 (b)
The system is the combination of water and air.
Solution:
water rises when the marble enters the water). This can also be deduced by considering that the net force exerted by the sinking marble on the water and the displacement of the boundary at the marble are in the same direction (downward). (c) The system is the combination of water, air, and marble.Solution:
sysis zero, becausewlabis zero (there is no displacement of the system boundary in the lab frame). sum of the internal energy change of the marble and the internal energy change of the water and air. In parts (a) and (b) these changes were found to be zero and positive, respectively.gasTextD300:0K
p extD1:00barpD3:00barVD0:500m3
TD300:0K
porous plug pistonFigure 3
3.3Figure
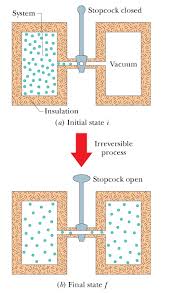
3 shows the initial state of an apparatus consisting of an ideal gas in a bulb, a stopcock, a porous plug, and a cylinder containing a frictionless piston. The walls are diathermal, and the surroundings are at a constant temperature of300:0K and a constant pressure of1:00bar. When the stopcock is opened, the gas diffuses slowly through the porous plug, and the piston moves slowly to the right. The process ends when the pressures are equalized and the piston stops moving. Thesystemis the gas. Assume that during the process the temperature through- out the system differs only infinitesimally from300:0K and the pressure on both sides of the piston differs only infinitesimally from1:00bar. (a) Which of these terms correctly describes the process: isothermal, isobaric, isochoric, reversible, irreversible?Solution:
The process is isothermal and irreversible, but not isobaric, isochoric, or reversible. Note that the pressure gradient across the porous plug prevents intermediate states of the process from being equilibrium states, and keeps the process from being reversible; there is no infinitesimal change that can reverse the motion of the piston. (b)Calculateqandw.
Solution:
BecauseTis constant and the gas is ideal, the relationp1V1Dp2V2holds, and the final volume is found from 10 V2Dp1V1
p2D.3:00bar/.0:500m3/
1:00barD1:50m3
The work must be calculated from the pressure at the moving portion of the boundary (the inner surface of the piston); this is a constant pressure of1:00bar: wD Z V2 V1pdVD p.V2V1/D .1:00105Pa/.1:500:500/m3
D 1:00105J
3.4 Consider a horizontal cylinder-and-piston device similar to the one shown in Fig. 3.5 on page 72. The piston has massm. The cylinder wall is diathermal and is in thermal contact with a heat reservoir of temperatureText. Thesystemis an amountnof an ideal gas confined in the cylinder by the piston. The initial state of the system is an equilibrium state described byp1andTDText. There is a constant external pressurepext, equal to twicep1, that supplies a constant external force on the piston. When the piston is released, it begins to move to the left to compress the gas. Make the idealized assumptions that (1) the piston moves with negligible friction; and (2) the gas remains practically uniform (because the piston is massive and its motion is slow) and has a practically constant temperatureTDText(because temperature equilibration is rapid). (a)
Describe the resulting process.
Solution:
The piston will oscillate; the gas volume will change back and forth between the initial valueV1and a minimum valueV2. (b) Describe how you could calculatewandqduring the period needed for the piston velocity to become zero again.Solution:
The relation betweenV1andV2is found by equating the work done on the gas by the piston,nRTln.V2=V1/, to the work done on the piston by the external pressure, pext.V2V1/, wherepextis given bypextD2p1D2nRT=V1. The result is V2D0:2032V1,wD1:5936nRT,qD wD 1:5936nRT.
(c) Calculatewandqduring this period for0:500mol gas at300K.Solution:
qD wD 1:99103J. 3.5 This problem is designed to test the assertion on page 60that for typical thermodynamic pro- cesses in which the elevation of the center of mass changes, it is usually a good approximation to setwequal towlab. The cylinder shown in Fig. 4 on the next page has a vertical orientation, so the elevation of the center of mass of the gas confined by the piston changes as the piston slides up or down. Thesystemis the gas. Assume the gas is nitrogen (MD28:0gmol1) at
300K, and initially the vertical lengthlof the gas column is one meter. Treat the nitrogen as
an ideal gas, use a center-of-mass local frame, and take the center of mass to be at the mid- point of the gas column. Find the difference between the values ofwandwlab, expressed as a percentage ofw, when the gas is expanded reversibly and isothermally to twice its initial volume.Solution:
Use Eq.
3.1.4 :wwlabD 1 211gasl
Figure 4
2 l1 2 D2l1 2 l1 2 D1 2 l1; thereforewwlabD 1 2 mgl1.From Eq.
3.5.1 , which assumes the local frame is a lab frame: w labD nRTlnV2 V1D nRTln2.
Use these relations to obtainwDwlab1
2 mgl1D nRTln21 2 mgl1. wwlab w D1 2 mgl1 nRTln21 2 mgl1D12nRTln2
mgl 1C1D12RTln2
Mgl 1C1 D 1 .2/.8:3145JK1mol1/.300K/.ln2/ .28:0103kgmol1)(9.81ms2)(1m)C1D7:9105 which is0:0079%. weightvacuum ideal gas hFigure 5
3.6Figure
5 shows an ideal gas confined by a frictionless piston in a vertical cylinder. Thesystem is the gas, and the boundary is adiabatic. The downward force on the piston can be varied by changing the weight on top of it. 12 (a) Show that when the system is in an equilibrium state, the gas pressure is given bypD mgh=Vwheremis the combined mass of the piston and weight,gis the acceleration of free fall, andhis the elevation of the piston shown in the figure.Solution:
The piston must be stationary in order for the system to be in an equilibrium state. Therefore the net force on the piston is zero:pAsmgD0(whereAsis the cross-section area of the cylinder). This givespDmg=AsDmg=.V=h/Dmgh=V. (b) Initially the combined mass of the piston and weight ism1, the piston is at heighth1, and the system is in an equilibrium state with conditionsp1andV1. The initial temperature isT1Dp1V1=nR. Suppose that an additional weight is suddenly placed on the piston, so thatmincreases fromm1tom2, causing the piston to sink and the gas to be com- pressed adiabatically and spontaneously. Pressure gradients in the gas, a form of friction, eventually cause the piston to come to rest at a final positionh2. Find the final volume, V2, as a function ofp1,p2,V1, andCV. (Assume that the heat capacity of the gas,CV,
is independent of temperature.) Hint: The potential energy of the surroundings changes and end of the process, and the boundary is adiabatic, the internal energy of the gas mustSolution:
Equate the two expressions and substituteT1Dp1V1=nRandT2Dp2V2=nR: .CV=nR/.p2V2p1V1/D p2.V2V1/
Solve forV2:V2DCVp1CnRp2
p2.CVCnR/V1
(c) It might seem that by making the weight placed on the piston sufficiently large,V2could be made as close to zero as desired. Actually, however, this is not the case. Find ex- pressions forV2andT2in the limit asm2approaches infinity, and evaluateV2=V1in this limit if the heat capacity isCVD.3=2/nR(the value for an ideal monatomic gas at room temperature).Solution:
Sincep2is equal tom2g=As,p2must approach1asm2approaches1. In theexpression forV2, the termCVp1becomes negligible asp2approaches1; thenp2cancels from the numerator and denominator giving
V 2!nR CVCnRV1
The relationT2Dp2V2=nRshows that with a finite limiting value ofV2,T2must approach1asp2does. IfCVequals.3=2/nR, thenV2=V1approaches2=5D0:4.
3.7The solid curve in Fig.
3.7 on page 80shows the path of a reversible adiabatic expansion or compression of a fixed amount of an ideal gas. Information about the gas is given in the figure caption. For compression along this path, starting atVD0:3000dm3andTD300:0K and ending atVD0:1000dm3, find the final temperature to0:1K and the work.
Solution:
C 13 T2DT1V1
V 2 nR=CVD.300:0K/.3:000/.1=1:500/D624:0Kquotesdbs_dbs14.pdfusesText_20[PDF] second order differential equation rlc circuit
[PDF] second order low pass filter
[PDF] second trimester bleeding differential diagnosis
[PDF] second year computer engineering syllabus pune university 2019 20
[PDF] secondary amine reaction with hcl
[PDF] secondary amine reaction with nano2 and hcl
[PDF] secondary amine reaction with water
[PDF] secondary colors of light
[PDF] secondary consumers are eaten by larger
[PDF] secondary consumers in the desert
[PDF] secondary consumers in the ocean
[PDF] secondary consumers in the rainforest
[PDF] secondary consumers in the savanna
[PDF] secondary consumers in the tundra

