 Questions - C08 Separation Techniques II - Section 2 Chemistry
Questions - C08 Separation Techniques II - Section 2 Chemistry
C8: Separation Techniques II - Question by Topic. (Mark Scheme and explanations Answers and Explanations. 1. The answer is A. Common sulfates nitrates and ...
 Separation Techniques Exam Style Questions 1
Separation Techniques Exam Style Questions 1
In the space below draw and label the apparatus required to separate a soluble substance from an insoluble substance. A. B. Page 2. Answers. 1. Distillation is
 Separation Techniques Chemistry Questions with Solutions
Separation Techniques Chemistry Questions with Solutions
Which method can be used to separate a mixture of two solids? Answer: All mixtures of two solid substances can be separated using: ○ Using a suitable solvent.
 SEPARATION TECHNIQUES – IGCSE (MCQS)
SEPARATION TECHNIQUES – IGCSE (MCQS)
SEPARATION TECHNIQUES – IGCSE (MCQS). 0620_s/12/qp11. 0620_s/11/qp11. 0620_s/11/qp11. Page 2. 0620_s/10/qp11. 0620_s/09/qp11. 0620_s/08/qp1. Page 3
 National Quali cations 2018 X707/77/02 Biology Section 1
National Quali cations 2018 X707/77/02 Biology Section 1
15 May 2018 Record your answers on the answer grid on page 03 of your question and answer booklet. ... Protein separation technique. Centrifugation. Gel ...
 Questions and Answers
Questions and Answers
4 Apr 2017 MiFID I required firms to “take all reasonable steps to obtain when executing orders
 Separating Mixtures – Exam Questions - Ms Finnegans Science
Separating Mixtures – Exam Questions - Ms Finnegans Science
Questions. Page 2. 2012 - Higher. Paper chromatography was used to find the Name the separation technique shown in the diagram. In which labelled part ...
 Home learning activities Subject Science Year Group Year 8 Unit of
Home learning activities Subject Science Year Group Year 8 Unit of
Complete the exam question on 'Mixtures and Separation Techniques'. Use the mark scheme (once you have tried the question) to mark your answers carefully
 Spring Term 1 Year 8 Separation techniques and Energy Extended
Spring Term 1 Year 8 Separation techniques and Energy Extended
Year 8 Separation techniques and Energy. Extended Homework Assignment. Name Look at the table of information below and answer the questions. Food or ...
 Solution Using Separation of Variables
Solution Using Separation of Variables
However the separation of variables technique does give some useful solutions to Now write down the final answer for u(x
 Questions - C08 Separation Techniques II - Section 2 Chemistry
Questions - C08 Separation Techniques II - Section 2 Chemistry
C8: Separation Techniques II - Question by Topic What is the best method to perform this separation? ... Answers and Explanations. 1. The answer is A.
 Spring Term 1 Year 8 Separation techniques and Energy Extended
Spring Term 1 Year 8 Separation techniques and Energy Extended
1 Match the name of the separation technique to the correct experimental set up below. Look at the table of information below and answer the questions.
 Separation purification and identification of the components of a
Separation purification and identification of the components of a
Hints for the answers to the proposed questions and topics to discussion: principles and practice of purification and separation techniques in Organic ...
 Separating Mixtures – Exam Questions - Ms Finnegans Science
Separating Mixtures – Exam Questions - Ms Finnegans Science
Name the separation technique shown in the diagram. In which labelled part would you expect to find most of the dye at the end of the experiment?
 Separation Techniques
Separation Techniques
Question: Why is table salt (sodium chloride) added to water that is used for cooking? Answer: The sodium chloride is an impurity that will increase the boiling
 GCSE T?ME
GCSE T?ME
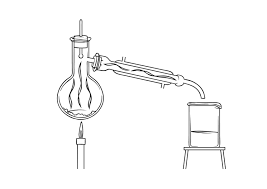
Separation Techniques Exam Style Questions 1 Answers. 1. Distillation is a method that can be used to obtain drinking water from a sample of salt water.
 Multiple choice questions on separation techniques pdf
Multiple choice questions on separation techniques pdf
Multiple Choice Question Separation Techniques (MCQ) the separation techniques quiz answers pdf to study grade 6 science for online undergraduate courses.
 Sample Practice Questions Answers
Sample Practice Questions Answers
https://onlinelibrary.wiley.com/doi/pdf/10.1002/9781119204206.oth01
 Lab #2
Lab #2
Techniques useful for the separation of mixtures include the following: DISTILLATION is the purification of a liquid techniques shown as question marks.
 Year 8 Science Distance Learning Quiz and Learn Booklet Part 1
Year 8 Science Distance Learning Quiz and Learn Booklet Part 1
20 May 2020 Practice questions and answers for you to complete and self mark ... ANSWERS: Separation techniques: Week 4 (22nd June) ...
 Unit 7 SEPARATION TECHNIQUES Question Bank - Miss Pirulli
Unit 7 SEPARATION TECHNIQUES Question Bank - Miss Pirulli
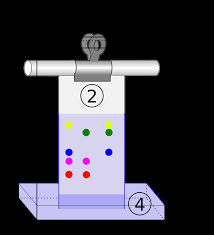
SEPARATION TECHNIQUES A)chromatography B)electrolysis C)distillation D)titration 23 Given the diagram representing a process being used to separate the colored dyes in food coloring: Which process is represented by this diagram? A)elements with identical boiling points B)elements with different boiling points C)compounds with identical boiling
 SEPARATION TECHNIQUES - Sepali's Chemistry Guide
SEPARATION TECHNIQUES - Sepali's Chemistry Guide
Separation Techniques Exam Style Questions 1 1 Distillation is a method that can be used to obtain drinking water from a sample of salt water Two different examples of distillation apparatus are shown below • Which apparatus A or B will produce the drinking water the quickest? Give a reason for your choice
 SEPARATION TECHNIQUES - Sepali's Chemistry Guide
SEPARATION TECHNIQUES - Sepali's Chemistry Guide
SEPARATION TECHNIQUES If a substance does not dissolve in a solvent we say that it is insoluble For example sand does not dissolve in water – it is insoluble Filtration is a method for separating an insoluble solid from a liquid When a mixture of sand and water is filtered: • the sand stays behind in the filter paper (it becomes the
 Separating Mixtures - CSIRO
Separating Mixtures - CSIRO
substances that can be separated using a range of techniques (ACSSU113) -recognising the differences between pure substances and mixtures and identifying examples of each -identifying the solvent and solute in solutions -investigating and using a range of physical separation techniques such as filtration
 Unit 7 SEPARATION TECHNIQUES Question Bank
Unit 7 SEPARATION TECHNIQUES Question Bank
SEPARATION TECHNIQUES A)chromatography B)electrolysis C)distillation D)titration 23 Given the diagram representing a process being used to separate the colored dyes in food coloring: Which process is represented by this diagram? A)elements with identical boiling points B)elements with different boiling points C)compounds with identical boiling
 Searches related to separation techniques questions and answers filetype:pdf
Searches related to separation techniques questions and answers filetype:pdf
What name is given to this separation technique? The salt and water was collected at X Explain why the insoluble impurities (dirt) were held at Y 2011 - Ordinary To get the salt from the mixture of salt and water the water was removed This could be done by either evaporation or distillation
How can mixtures of liquids be separated according to their properties?
- Mixtures of liquids can be separated according to their properties. The technique used depends on whether the liquids dissolve in each other, and so are miscible, or if they are immiscible. Fractional distillation is a technique used to separate miscible liquids according to their boiling points.
Which mixtures are suitable for separation by chromatography?
- Mixtures that are suitable for separation by chromatography include inks, dyes and colouring agents in food. Simple chromatography is carried out on paper. A spot of the mixture is placed near the bottom of a piece of chromatography paper and the paper is then placed upright in a suitable solvent, eg water.
How do you separate a soluble solid from a liquid?
- Pour the mixture through the filter funnel. Let the water drain and leave the insoluble solid to dry. Eg. Separating sand from Salt water. Evaporation is used to separate a soluble solid from a liquid. For example, copper sulfate is soluble in water – its crystals dissolve in water to form copper sulfate solution.
Separating Mixtures
Author: Ms Pam Burt
This resource was developed as a result of participation in CSIRO"s teacher professional learning program, Teacher Researcher in Partnership Program. © Separating Mixtures (created by Pamela Burt) (2019). Copyright owned by West Moreton Anglican College. Except as otherwise noted, this work is licenced under the Creative Commons Attribution4.0 International Licence. To view a copy of this licence, visit
UNIT PLAN: Year 7, Science unit, 2019
TRiPP Project
Overview
Compositional analysis of apple and grape pomace, and soil and river water samples with researcher Dr Avinash Karpe (Jan 2019)
- Dr Karpe"s work focuses on deriving useful products from winery biomass using fungi.The aim is to reduce the amount of biomass that goes to landfill and instead convert it to useful products. The project experience involved processing and GC-MS analysis of apple,
grape and soil samples.Nature of problem Winery waste (pomace) is being disposed of in landfill. Alternative solutions for this issue are being investigated.
Links to Unit Students will learn about separation techniques and be introduced in a very basic way to gas chromatography- mass spectroscopy as a way to
separate and identify components of a mixture. They will mathematically analyse some of the data collected from my TR
iPP experience. Theywill identify the benefits to society of applying scientific and technological understanding to solve an environmental issue.
YearLevel:
Subject: Unit Title: Separating mixtures Term: - 7 ScienceMathematics
Technology
Achievement Standard
Science
By the end of Year 7, students
- describe techniques to separate pure substances from mixtures- analyse how the sustainable use of resources depends on the way they are formed and cycle through Earth systems
- describe situations where scientific knowledge from different science disciplines and diverse cultures has been use
d to solve a real-world problem- communicate their ideas, methods and findings using scientific language and appropriate representations.
Mathematics
By the end of Year 7, students
- identify issues involving the collection of continuous data - calculate mean for data setsTechnology
By the end of Year 8, students explain how social, ethical, technical and sustainabilityconsiderations influence the design of innovative and enterprising solutions to meet a range of present and future needs.
Learning Objective
To apply understanding of mixtures and separation techniques to the context of biomass waste reduction and process and interpret secondary data
using statistical techniques.Learning Outcomes
Students will be able to distinguish between elements, compounds and mixtures as well as homogenous and heterogenous mixtures. They will be able
to choose and perform separation techniques appropriate to a mixture provided. Students will be able to understand how chromatography can be used
to separate solutions and that GC-MS is an advanced technique that extends on their understanding of paper chromatography. They will be able to calculate percentages and mean from data.
UNIT PLAN: Year 7, Science unit, 2019
General Capabilities
Literacy
Numeracy
Information and communication technology capabilityCritical and creative thinking
Personal and social capability
Ethical understanding
Intercultural understanding
ICT Capability Personal & Social Capability Ethical Understanding Intercultural UnderstandingIn the Australian Curriculum:
Recognise intellectual property
Define and plan information searches
Locate, generate and access data and information
Select and valuate data and information
Generate solutions to challenges and learning area tasksIn the Australian Curriculum:
Develop reflective practice
Develop self-discipline and set goals
Work independently and show initiative
Appreciate diverse perspectives
Contribute to civil society
Communicate effectively
Work collaboratively
Make decisions
Cross Curriculum Priorities related to Humanitarian Engineering ABTI Histories & Culture Asia & Australia"s Engagement with Asia Sustainability1. The interdependent and dynamic nature of systems that support all life
on Earth and our collective wellbeing.2. A diversity of world views on ecosystems, values and social justice
3. Building capacities for
thinking and acting in ways that are necessary to create a more sustainable future. General Capabilities related to Humanitarian Engineering Literacy use a wide range of new specialist and topic vocabulary to contribute to the specificity, authority and abstraction of textsCritical and
Creative
Thinking
Inquiring: identifying, exploring and
organising information and ideas,Reflecting on thinking and processes,
Analysing
and evaluating reasoning and procedures Numeracy compare, interpret and assess the effectiveness of different data displays of the same informationUNIT PLAN: Year 7, Science unit, 2019
Australian Curriculum content descriptors and elaborationsAC Content Descriptors Elaborations
Mixtures, including solutions, contain a combination of pure substances that can be separated using a range of techniques (ACSSU113) -recognising the differences between pure substances and mixtures and identifying examples of each -identifying the solvent and solute in solutions -investigating and using a range of physical separation techniques such as filtration, decantation, evaporation, crystallisation, chromatography and distillation Identify and investigate issues involving numerical data collected from primary and secondary sources (ACMSP169) -using authentic problems to express quantities as percentages of other amounts Calculate mean, median, mode and range for sets of data. Interpret these statistics in the context of data (ACMSP171) -understanding that summarising data by calculating measures of centre and spread can help make sense of the dataDIMENSION 2
- VOCABULARY EXPLICITLY TAUGHT COGNITIVE VERBS ESSENTIAL VOCABULARY Retrieval and Comprehension Pure substance, Mixture, Solution, Dissolve, Solute, Solvent, Soluble, Insoluble, Homogenous, Heterogenous, Separate, Paper chromatography, Gas chromatography, Stationary phase, Mobile phase, Solvent front,Retention factor, Mass spectrometry,
Calculate, Describe, Explain,
Analysis
Analyse, Interpret, Compare
Knowledge Utilisation
Experiment, Justify
UNIT PLAN: Year 7, Science unit, 2019
DIMENSIONS OF LEARNING
Dimension 3 (Extend & Refine Knowledge)
տ Classifying
տ Abstracting
տ Constructing support
Dimension 4 (Use Knowledge Meaningfully)
տ Systems analysis
Dimension 5 (Habits of Mind)
Questioning and problem solving: Adopt a
questioning and inquisitive mindset. Nothing is taken for granted and questions are the key to a better understanding.Thinking flexibly: Be flexible with your thoughts
and be ready to try different alternatives and options.Applying past knowledge to new situations:
Draw on your prior knowledge to enhance your
present learning experiences. Maintain a connection between your past knowledge and your actual learning. Responding with wonderment and awe: Enjoy your learning and have fun learning more.Taking responsible risks: Be adventuresome and
try new things constantly.Choose an item.
Persisting: Persevere in you what you do and
keep focused. Thinking interdependently: Develop team work skills and know how to work collaboratively with others.Managing impulsivity: Take your time and think
before you act.Keep thoughtful and deliberative
UNIT PLAN: Year 7, Science unit, 2019
LESSON
LEARNING
GOALS LESSON CONTENT RESOURCES DIFFERENTIATION STRATEGIES 1Elements,
compounds and mixtures1. Types of mixtures
Mixture ppt
Source : www.northallegheny.org
Accessed: 5/2/20
Extra scaffolding
Break activities into manageable
chunksSpecific grouping
Monitor work output
in incrementsEncourage involvement
Minimise choices
2Separating
mixtures2. Separation techniques
3. Separating mixtures prac
Separation techniques ppt
Source: bgreyson.weebly.com
Accessed: 5/2/20
Separating mixtures worksheet
3 Chromatography
1. Principles of chromatography
2. Paper chromatography prac
Principles of chromatography ppt
Source: seaver-faculty.pepperdine.edu
Accessed: 5/2/20
Paper chromatography worksheet
4Applications of
chromatography1. Winery biomass waste
2. Using chromatography to work a solution
Use of biomass ppt
Separating Mixtures Practical
Station 1 - Centrifugation
Materials:
Benchtop manual centrifuge and tubes
Calcium hydroxide
Vegetable oil
Method:
1. Add a small amount of calcium hydroxide powder to a centrifuge tube. Add water
until approx. 2cm from the top. Cap and shake until fully mixed.2. Place the tube in the centrifuge.
3. Add equal amounts of oil and water to the second tube. Cap and shake until fully
mixed.4. Place the tube in the centrifuge opposite to the calcium hydroxide tube. This will keep the apparatus balanced.
5. Turn the handle gently and spin the samples for a few minutes.
6. Carefully remove the tubes and observe.
7. When finished leave the filled tubes for the next group.
Questions:
1. What physical property does centrifugation use to separate mixtures?
2. Which substances were pulled to the bottom of the centrifuge tubes?
3. What does this tell you about them?
4. What type of mixture can be separated using centrifugation? Homogenous/Heterogenous?
Station 2 - Distillation
Materials:
Distillation apparatus
Coloured water
This is a demonstration only station. Do not touch the equipment. Observe what is happening and answer the questions below. If the amount of impure liquid is getting low, tell your teacher.Questions
1. What physical property does distillation use to separate mixtures?
2. Where does evaporation take place?
3. Where does condensation take place?
4. Which component of the mixture (dye or water) is the distilled liquid?
5. What does this suggest about it compared to the other component?
6. What type of mixture can be separated using distillation? Homogenous/Heterogenous
Station 3
- Stacked sievingMaterials:
6 disposable cups
Hole poking tools (5 sizes)
Rock mixture
Method:
1. Using the tools provided, poke holes (from the inside to outside) in the cup in 5 different sizes.
Don"t put holes in the 6
th cup.2. Stack the cups so that the largest holes are on the top, decreasing in size to the smallest holes
and then the cup with no holes.3. Pour a sample of the rock mixture into the top cup.
4. Lift the first cup slightly and shake it so the rocks that can fit through the holes will move into
the cup below. When only the largest rocks remain, take the cup out and put it to the side.5. Repeat the process with each cup in turn.
6. At the end you should hopefully have 6 cups with particles of different sizes in them.
7. Tip the rock sample back into the original container and mix well. Leave the setup for the next
group.Questions
1. What physical property does stacked sieving use to separate mixtures?
2. Why must the cups be stacked in this order? What would happen if they weren"t?
3. Were all of the rocks in each layer of a similar size? If they weren"t, how could you modify the design to make this happen?
4. What type of mixture can be separated using stacked sieving? Homogenous/Heterogenous
Paper Chromatography Lab
Student worksheet
Learning objectives:
To use paper chromatography to separate the components of a mixture. To calculate the retention factor (Rf) for each component. To interpret the Rf values and compare the solubility of the components. To compare paper chromatography with gas chromatography.Safety:
Wear safety googles at all times during the experiment.Materials:
Beaker or similar container
Pencil
RulerSticky tape
Strip of filter paper 5cm wide
Water - Water soluble marker (black works well)
Method:
1. Add 1cm of water to the beaker.
2. Rule a straight line with the pencil across the strip of paper
about 1cm from the bottom.3. Draw a spot with the marker on the ruler line.
4. Tape the top of the strip of paper to a pencil so that the end
of the paper hangs touches the water but the spot is not submerged.5. Let sit until the component colours have travelled up the paper
approximately 2/3 of the way.6. Remove from the cup, mark the solvent front in pencil immediately
and leave to dry.Analysis:
1. Measure the distance from the pencil line to the centre of each band of colour.
2. Measure the distance from the pencil line to the solvent front.
3. Use the formula below to calculate the retention factor for each component.
Rf =Band colour
Distance travelled
Solvent front
quotesdbs_dbs4.pdfusesText_8[PDF] sept cent euros en lettre
[PDF] september 11 in history
[PDF] september 2016 movies in theaters
[PDF] september 2017 movies in theaters
[PDF] sequel viewpoint case statement
[PDF] sequel viewpoint date functions
[PDF] sequel viewpoint script examples
[PDF] sequence control statements in java
[PDF] sequence of basic feasible solution
[PDF] sequences (xn) and (yn)
[PDF] sequencing speech therapy goals
[PDF] serial dilution and plating
[PDF] serial dilution calculations microbiology
[PDF] serial dilution calculator cells
