 Well What Will We Drink
Well What Will We Drink
www.epa.gov/safewater/consumer/pdf/mcl.pdf. Individual Water Supply Wells Sample Students Answers: ppm ppb
 PREPARING SOLUTIONS AND MAKING DILUTIONS
PREPARING SOLUTIONS AND MAKING DILUTIONS
Final dilution factor (DF) = DF1 * DF2 * DF3 etc. Example: In the microbiology lab BIOLOGY 201 the students perform a three step 1:100 serial dilution of a
 Beers Law: Determining the Concentration of a Solution
Beers Law: Determining the Concentration of a Solution
Don't forget: Attach a copy of your graph to the lab report sheets. Page 8 This experiment covers the concept of molarity dilution
 National Functional Guidelines for Inorganic Superfund Methods
National Functional Guidelines for Inorganic Superfund Methods
Nov 2 2020 Laboratory serial dilution reports (if available)
 lab 9 serial dilution pour plates
lab 9 serial dilution pour plates
https://people.fmarion.edu/gpryor/LAB%209%20BIO%20215L.pdf
 Laboratory Math II: Solutions and Dilutions
Laboratory Math II: Solutions and Dilutions
Molarity is used to report molecules of a substance per unit volume. While serial dilutions are a great way to save on both reagents and space in the ...
 Serial Dilution Lab
Serial Dilution Lab
This exercise is intended to familiarize us with using scientific notation (to express big and small number easily) and the process of using serial dilutions to
 lab 9 serial dilution pour plates
lab 9 serial dilution pour plates
https://people.fmarion.edu/gpryor/LAB%209%20BIO%20215L.pdf
 PREPARING SOLUTIONS AND MAKING DILUTIONS
PREPARING SOLUTIONS AND MAKING DILUTIONS
Final dilution factor (DF) = DF1 * DF2 * DF3 etc. Example: In the microbiology lab BIOLOGY 201 the students perform a three step 1:100 serial dilution of a
 Suggested Reporting Language for Syphilis Serological Testing
Suggested Reporting Language for Syphilis Serological Testing
Guidance on Laboratory Reporting for Surveillance . Measurement of antibodies (i.e. nontreponemal) through serial dilution of the patient specimen to.
 Lab Report
Lab Report
Individual lab reports sharing same data. The lab report and marks can be viewed
 Laboratory Exercises in Microbiology: Discovering the Unseen
Laboratory Exercises in Microbiology: Discovering the Unseen
Scientific Notation and Serial Dilution …………………………..………… 177 Lab 1 of the lab manual. Report any problems with your microscope to your instructor.
 Lab #4 SPECTROPHOTOMETRY
Lab #4 SPECTROPHOTOMETRY
Serial Dilutions (Background). A dilution series is a succession of step dilutions each with the same dilution factor
 Lab Math Solutions Dilutions
Lab Math Solutions Dilutions
https://www.aphl.org/programs/newborn_screening/Documents/2016%20Molecular%20Workshop/5%20-%20Lab%20Math%20%20Molarity.pdf
 BIL 151 - General Biology Laboratory Dilutions: Simple and Serial
BIL 151 - General Biology Laboratory Dilutions: Simple and Serial
Serial dilutions can be used to prepare solutions of any desired concentration no matter how dilute You will perform a simple exercise to be sure you understand how to do this as it will be important in your future biological research endeavors In a pinch here’s a Serial Dilution Calculator: https://www aatbio com/tools/serial-dilution
 LAB 9 SERIAL DILUTION POUR PLATES AND ENUMERATION OF BACTERIA
LAB 9 SERIAL DILUTION POUR PLATES AND ENUMERATION OF BACTERIA
A serial dilution is the dilution of a sample in 10-fold dilutions As shown in the illustration below it begins when 1 mL of the bacterial sample is added to 9 mL and it is mixed together (creating a 10-1 dilution) Then 1 mL from that mixture is added to 9 mL and it is mixed together (a 10-2 dilution) That
 How to Do Serial Dilutions: 9 Steps (with Pictures) - wikiHow
How to Do Serial Dilutions: 9 Steps (with Pictures) - wikiHow
prepare a dilute solution from a more concentrated one perform serial dilutions use volumetric and Mohr pipets and a volumetric flask DISCUSSION Solutions are an important part of chemistry In this lab you will practice preparing solutions of different concentrations
 Microbiology BIOL 275 DILUTIONS - University of Pennsylvania
Microbiology BIOL 275 DILUTIONS - University of Pennsylvania
The serum dilution is the amount of serum in the amount of total solution; hence this is a 5/25 serum dilution which would equal a 1/5 dilution Titer The titer is the smallest amount or concentration that will produce a particular effect or endpoint
 LAB 8 SERIAL DILUTION POUR PLATES AND ENUMERATION OF BACTERIA
LAB 8 SERIAL DILUTION POUR PLATES AND ENUMERATION OF BACTERIA
A serial dilution is the dilution of a sample in 10-fold dilutions As shown in the illustration below it begins when 1 mL of the bacterial sample is added to 9 mL and it is mixed together (creating a 10-1 dilution) Then 1 mL from that -mixture is added to 9 mL and it is mixed together (a 10 2 dilution) That
 Searches related to serial dilution lab report pdf filetype:pdf
Searches related to serial dilution lab report pdf filetype:pdf
Activity 1 1 5 Student Resource Sheet Serial Dilutions In the lab scientists often need to make dilutions of the same solution Producing samples with different concentrations in a series is more time efficient than trying to prepare each sample one by one A serial dilution is a stepwise dilution of a substance in solution
How to calculate the serial dilution?
- Calculate the final dilution ratio in a serial dilution. The total dilution ratio can be determined by multiplying the dilution factor of each step leading up to the final step. This can be mathematically illustrated with the equation D t = D 1 x D 2 x D 3 x … x D n where D t is the total dilution factor and D n is the dilution ratio.
Why is serial dilution necessary?
- serial dilutions are necessary to count cfus because they estimate the concentration (the number of bacteria or colonies) of a sample by diluting the original culture until you have a serial dilution that results in a countable number of colonies on a plate (usually between 30 to 300 cfus), which will be used to determine the cfu/ml count of the …
What does serial dilution mean?
- In a single and very simple word, Serial dilution is a laboratory technique, in which a stepwise dilution process is performed on a solution with an associated dilution factor. In the laboratory, this method is used to decrease the counts of cells within a culture to simplify the operation.
Experiment 16
The Solution is Dilution
OUTCOMES
Upon completion of this lab, the student should be able to proficiently calculate molarities for solutions. prepare a solution of known concentration. prepare a dilute solution from a more concentrated one. perform serial dilutions. use volumetric and Mohr pipets and a volumetric flask.DISCUSSION
Solutions are an important part of chemistry. In this lab you will practice preparing solutions of different concentrations. The amount of solute that is dissolved in a given quantity of solvent is expressed as the concentration of the solution. A dilute solution contains only a small amount of solute in a given amount of solution. The units chemists use most often to describe concentration of solutions are molarity units. The molarity, M, of a solution is the number of moles of solute in one liter of solution. To determine the molarity of a solution, the following equation can be used:Molarity (M) = solutionof Liters
soluteof moles Example 1: How would 500.0 mL of a 0.6000 M NaCl solution be prepared? Since NaCl is a solid, its mass must be measured on a balance. The first step is to calculate the mass of solute needed.The molar mass for NaCl is 58.44 g/mol.
NaCl mol
1NaCl g
58.44solution L 1
NaCl mol 0.6000
solution mL 1000 solution L 1 solution mL 500.0 = 17.53 g NaCl Answer: Using a balance, measure out 17.53 g of NaCl into a small beaker and transfer it to a 500-mL volumetric flask. A funnel may be necessary to transfer the solid into the flask. Rinse the beaker and funnel with a small amount of deionized water into the flask. Then add
deionized water to the flask until it is about ½ full. Cap the flask and invert it several times to
dissolve the solid. Next, add water until the liquid is just below the etched line on the neck of the flask. Bring the water to the line by adding the last few drops of water drop-by-drop using either a wash bottle or dropper. Again, cap and invert the flask several times to ensure propermixing. The final volume of the solution is 500.0 mL. See Figure 1 on the next page. 16.1 F14 Edition
Figure 1. Preparing a solution of known concentration using a solid solute. Experiments often require a solution that is more dilute than the stock solution or solution on hand. The following equation can be used to determine the amount of concentrated solution needed to carry out the dilution:Molarity
concentrated sol ution × Volume concentrated solution = Molarity dilute solution × Volume dilute solutionThe equation is commonly written as: M
1V1 = M2V2
Example 2: Suppose that an experiment required that 500.0 mL of a 0.200 M NaCl solution be prepared from the 0.6000 M NaCl solution prepared in the first example. What volume of the concentrated (0.6000 M) solution would be needed?NaCl 6000.0
mL 500.0 NaCl 200.0 or 1 221 1 22
1 11 M M M VM V M VM M VM
167 mL
To prepare this solution, 167 mL of the
0.6000 M NaCl would be measured and
transferred into a 500-mL volumetric flask. Deionized water would then be added up to the etched line on the neck of the flask and mixed thoroughly.See Figure 2 to the right.
Figure 2. Diluting a solution.
16.2 F14 Edition
The previous example is one kind of dilution. A serial dilution is a dilution where a series of dilutions are conducted, each one may be one-tenth as concentrated as the previous. This procedure is repeated until the desired concentration is reached. Serial dilutions are commonly used in microbiology where the solution being diluted contains bacterial colonies. See Figure 3 below.To prepare solutions through
serial dilution, 1.00 mL of a stock solution is removed using a pipet and added to a 10 mL graduated cylinder. Water is added so that the final volume is 10.00 mL. The solution is mixed and then poured into test tube #2. To prepare the next solution, 1.00 mL of solution from test-tube #2 is removed using a pipet and added to a 10 mL graduated cylinder. Again, deionized water is added to the graduated cylinder until the final volume is 10.00 mL. The solution is mixed and then poured into test tube #3.1.0 mL 1.0 mL 1.0 mL 1.0 mL 1.0 mL
10.0 9.0 mL 9.0 mL 9.0 mL 9.0 mL 9.0 mL
mL H2O H2O H2O H2O H2O
1 2 3 4 5 6
StockSolution
Figure 3.
Serial Dilutions.
Note: Since volumes are not additive,
you will not actually be measuring 9.0 mL of water to add to the concentrated solution. Instead, the correct procedure is to use a pipet to measure 1.00 mL of concentrated solution into a 10 mL graduated cylinder, then add deionized water to the graduated cylinder so that the final volume of the diluted solution is precisely 10.00 mL. Recall the proper pipet procedure you learned in experiment 2. The pipet must be kept vertical at all times when it has liquid in it (to prevent liquid from getting into the pump). When dispensing from a pipet, you must either stop at a certain volume marking, or, on pipets with markings all the way down to the tip, you must allow the liquid to drain out by gravity only. In either case, forcing all of the liquid out of the pipet will dispense too much.16.3 F14 Edition
PROCEDURE
PART A. Preparing a 0.10 M sucrose solution in a volumetric flask.1. Check your calculations from question #2 in the prelab exercises. Calculate the mass of
sucrose, C12H22O11, required to make 100.0 mL of a 0.10 M solution. Measure out the
required amount of sucrose into a new plastic weighing boat.2. Transfer the sucrose into a 100 mL volumetric flask, using a wash bottle to rinse any solid
remaining on the weighing boat . Add water to the flask until it is one-half to two-thirds full.Add 10 drops of food coloring to the solution.
3. Cap the flask and invert the flask gently several times until the solid dissolves completely.
Use your thumb or forefinger to secure the cap on
to the flask while inverting. Do not shake the flask hard as the glass neck may brea k.4. Continue (slowly) adding deionized water until the water level is near the etched line on the
neck of the flask. Add water more carefully, drop-by-drop, until the bottom of the meniscus is on the etched line.5. Cap the flask and invert the flask gently 3 to 4 more times. Do not shake the flask hard as
the glass neck may break.6. Once the solution is thoroughly mixed and all solid has been dissolved, transfer it to the
labeled plastic bottle. Cap the bottle to prevent contamination or evaporation. This is the0.10 M sucrose stock solution.
PART B. Preparing Serial Dilutions.
1. Place 5 clean, dry test tubes in a test tube rack and label the test tubes 1-5 with labeling
tape.2. Fill a 250-mL beaker about half full with deionized water. This water will be used to rinse
the pipet after each use. This will ensure the pipet is clean and not a cause of contamination. Label this beaker as the "rinse beaker."3. Pour your 0.10 M sucrose stock solution into a 10-mL graduated cylinder. The final volume
of liquid in the graduated cylinder should be precisely 10.00 mL. Pour this solution into the first test tube. Rinse the graduated cylinder with deionized water. Invert the cylinder and try to get as much water out as possible.16.4 F14 Edition
4. Using a rinse beaker, rinse a 1 mL volumetric pipet, by drawing in water followed by a small
amount of the solution that you intend to use and then discard it into a labeled "waste" beaker. This "seasons" the pipet with the liquid that you will be using and removes traces of the liquid previously in the pipet. Use this procedure for rinsing the pipet for the remainder of the lab. Be careful not to draw liquid up into the pipet bulb. Discard the contents of your waste beaker.5. Using the clean sucrose pipet, transfer 1.00 mL of sucrose solution to the 10 mL graduated
cylinder. Add deionized water so that the final volume on the cylinder is 10.00 mL. Mix this solution by drawing a small amount of the solution into the pipet and returning the solution back to the cylinder, or alternatively, you may carefully use a clean stirring rod. Transfer this solution to test tube #2. Rinse the pipet in the rinse beaker and rinse the 10 mL graduated cy linder with deionized water.6. Repeat step 5 by adding 1.00 mL of the sucrose solution from test tube # 2 to the graduated
cylinder. Add deionized water so that the final volume is 10.00 mL. Agitate the solution and add this newly mixed solution to test tube #3. Repeat the dilution process for test tubes #4 and #5. Don't forget to rinse your pipet and graduated cylinder out between steps.7. Compare the color of the stock solution and each of the subsequent dilutions in test tubes
1-5. Rank these solutions in order of color intensity from the darkest to the lightest color
(the most concentrated solution will have the highest number).8. Rinse each test tube with deionized water.
PART C. Preparing Solutions of a Given Molarity.
1. Place the 5 labeled test tubes back into the test tube rack. Using the 10 mL graduated
cylinder, pour 10.00 mL of the stock solution into test tube #1. Record the necessary data in the data table for test tube #1.2. Rinse a graduated Mohr pipet. Use this pipet to add 3.80 mL of the sucrose stock solution to
the 10 mL graduated cylinder. Now fill the graduated cylinder to the 10 mL mark with deionized water. Mix the solution by agitating with the pipet or a clean stirring rod. Transfer this solution to test tube #2. Rinse the pipet and graduated cylinder as directed earlier. Record the necessary data in the chart for test tube #2.3. Again, using the graduated Mohr pipet, add 2.40 mL of sucrose stock solution to the 10 mL
graduated cylinder. Now fill the graduated cylinder to the 10 mL mark with deionized water.Mix the solution by agitating with the pipet
or a clean stirring rod. Transfer the solution to test tube #3. Rinse the pipet and graduated cylinder as directed earlier. Record the necessary data in the chart for test tube #3.16.5 F14 Edition
4. Using your calculations from question number 3 in the prelab, prepare 10.00 mL of a
0.050 M sucrose solution by dilution using the stock solution. Transfer this solution to test
tube #4 and record the necessary data for test tube #4.5. Using your calculations from question number 4 in the prelab, prepare 10.00 mL of a
0.015 M sucrose solution by dilution using the stock solution. Transfer this solution to test tube #5 and record the necessary data for test tube #5.6. Compare the color of the stock solution and each of the subsequent dilutions in test tubes
1-5. Rank these solutions in order of color intensity from the darkest to the lightest color
(the most concentrated solution will have the highest number).7. After completing the data table, wash the test tubes and graduated cylinder, and rinse with
deionized water. Invert the test tubes in the rack so that they dry for the next lab class. Make sure that you have washed and dried all your equipment (the insides of test tubes and graduated cylinders do not need to be dried ) so that it is scrupulously clean for the next lab group. Make sure balances and surrounding bench areas are clean as well.16.6 F14 Edition
Name__________________________________________ Lab Section_________PRELAB QUESTIONS
1. What is the molar mass of sucrose, C
12H22O11?
2. How many grams of sucrose would be needed to prepare 100. mL of a solution of 0.10 M
sucrose? Show your calculations.3. Calculate the volume of 0.10 M sucrose solution, in mL, that must be diluted to prepare
10.00 mL of a 0.050 M sucrose solution. Show your work.
16.7 F14 Edition
4. Calculate the volume of 0.10 M sucrose solution that must be diluted to prepare 10.00 mL
of a 0.015 M sucrose solution. Show your work.5. Look at the procedure for using a pipet. Why should you not force the last remaining amount of liquid out of the pipet?
16.8 F14 Edition
Name_________________________________________ Lab Section__________Lab Partner's Name______________________________
DATAPart B:
Test tube # 1 Test tube # 2 Test tube # 3 Test tube # 4 Test tube # 5 M1 x V 1 M 2 x V2 Concentration
of ConcentratedSolution 0.10 M
Volume (in mL)
of ConcentratedSolution added 10.00 mL
Concentration
of DiluteSolution 0.10 M
(no water was added to this test tube)Volume (in mL)
of DiluteSolution 10.00 mL
(no water was added to this test tube)Color observations
Rank solutions 1-5
lightest = 1 darkest = 5Part C:
Test tube # 1 Test tube # 2 Test tube # 3 Test tube # 4 Test tube # 5 M1 x V 1 M 2 x V2 Concentration
of ConcentratedSolution 0.10 M
Volume (in mL)
of ConcentratedSolution added 10.00 mL
Concentration
of DiluteSolution 0.10 M
(no water was added to this test tube)Volume (in mL)
of DiluteSolution 10.00 mL
(no water was added to this test tube)Color observations
Rank solutions 1-5
lightest = 1 darkest = 516.9 F14 Edition
POSTLAB QUESTIONS
1. Compare the concentrations of each of the serial dilutions to the color rankings. What is the
relationship between concentration and color intensity?2. Calculate the number of grams of potassium iodide (KI), needed to prepare 500.0 mL of a 0.125 M KI. Show your work.
3. Suppose you took 1.00 mL of the KI solution from question #2 above and diluted it to
50.0mL, what would be the concentration of the new solution? Show your work.
4. 25.0 mL of 0.50 M solution of NaOH was diluted to a final volume of 200.0 mL. What is the
new concentration of this solution?16.10 F14 Edition
quotesdbs_dbs11.pdfusesText_17[PDF] serializable is an class inside io package. say true or false.
[PDF] serif fonts for screen reading
[PDF] seronegative spondyloarthropathy hla b27 negative
[PDF] serpa holster
[PDF] serpa l2 tactical holsters
[PDF] serpa p365 holster
[PDF] serre chevalier ski pass
[PDF] sers plop
[PDF] server jobs portland maine craigslist
[PDF] server side scripting languages
[PDF] serverless architecture
[PDF] serverless architecture aws lambda
[PDF] service a honda accord 2008
[PDF] service a honda accord 2012
 DATA VALIDATION REPORT
DATA VALIDATION REPORT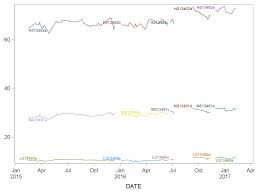 Ferritin Laboratory Procedure Manual
Ferritin Laboratory Procedure Manual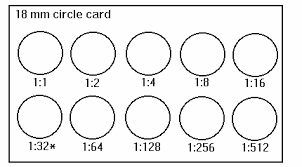 Lab 36 Syphillis Rapid Plasma Reagin
Lab 36 Syphillis Rapid Plasma Reagin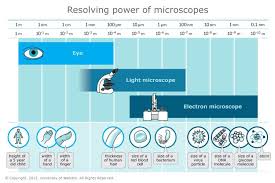 Laboratory Exercises in Microbiology: Discovering the Unseen
Laboratory Exercises in Microbiology: Discovering the Unseen BIOL 3702 Lab Exercise - Dilution Counting - 2019
BIOL 3702 Lab Exercise - Dilution Counting - 2019