 FEUILLE N 1 : ENSEMBLES RELATIONS
FEUILLE N 1 : ENSEMBLES RELATIONS
https://www.math.u-bordeaux.fr/~frgaunar/td1.pdf
 RELATION BINAIRE
RELATION BINAIRE
2. Décrire la classe ? de l'élément . 3. Pourquoi l'application On considère dans la suite de l'exercice que l'ensemble est ordonné par la relation . 2.
 ALGÈBRE Cours et Exercices Première Année LMD
ALGÈBRE Cours et Exercices Première Année LMD
2 Ensembles et Applications 3.1.1 Propriétés des relations binaires dans un en- ... La partie Solutions des exercices proposés que l'étudiant pourra.
 Chapter 10 Grand canonical ensemble
Chapter 10 Grand canonical ensemble
The grand canonical ensemble is a generalization of the canonical ensemble two systems are in equilibrium with the thermal ... This relation yields.
 1 Exemples simples de relations déquivalence 2 Construction de
1 Exemples simples de relations déquivalence 2 Construction de
Les exercices de cette section proposent plusieurs situations de ce type. Exercice 5. Soit E et F deux ensembles et f : E ? F une application. On définit le
 Relations et applications 1 Relation entre 2 ensembles. 2 Relation
Relations et applications 1 Relation entre 2 ensembles. 2 Relation
Exemples 1.4 Quel est le graphe dans l'exemple ii) précédent? Exercice 1.5 Soit sur les ensembles A = [0
 Chapter 5 Thermodynamic potentials
Chapter 5 Thermodynamic potentials
Maxwell relations. A Maxwell relation follows as discussed already in Sect. 4.4.2
 Relation déquivalence relation dordre
Relation déquivalence relation dordre
Indication ?. Correction ?. Vidéo ?. [000212]. 2 Relation d'ordre. Exercice 3. Soit (E?) un ensemble ordonné. On définit sur P(E){/0} la relation ? par.
 Logique ensembles et applications
Logique ensembles et applications
Exercice 12 ***IT. Montrer que les assertions suivantes sont équivalentes (f est une application d'un ensemble E dans lui-même) : 1. f est injective. 2.
 Ensembles Relations déquivalence
Ensembles Relations déquivalence
https://livres-mathematiques.fr/onewebmedia/L1-MI-arith-ch1.pdf
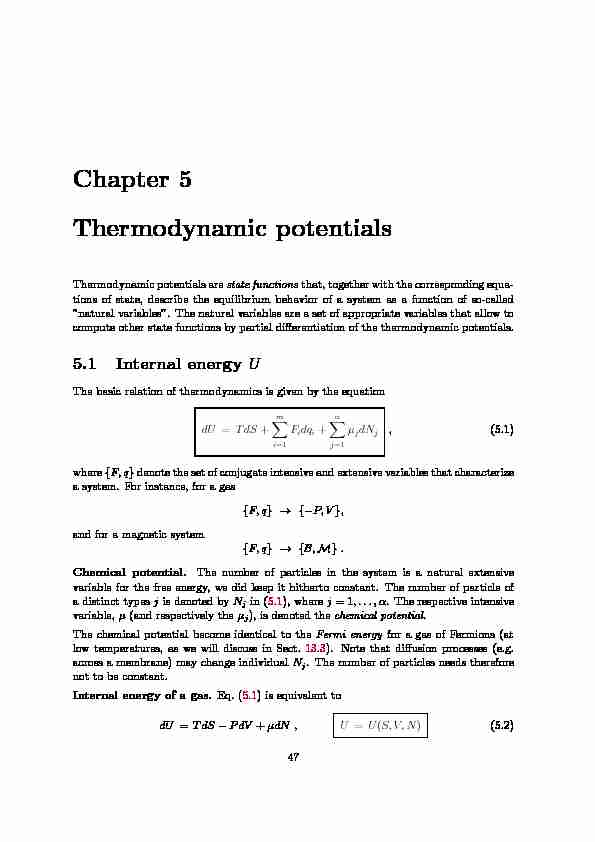
Chapter 5
Thermodynamic potentials
Thermodynamic potentials are
state functions that, together with the corresponding equa- tions of state, describe the equilibrium behavior of a system as a function of so-called "natural variables". The natural variables are a set of appropriate variables that allow to compute other state functions by partial di ff erentiation of the thermodynamic potentials.5.1 Internal energy
U The basic relation of thermodynamics is given by the equation dU TdS m i =1 F i dq i j =1 j dN j (5.1) where F,q denote the set of conjugate intensive and extensive variables that characterize a system. For instance, for a gas F,q P,V and for a magnetic system F,q {B M}Chemical potential.
The number of particles in the system is a natural extensive variable for the free energy, we did keep it hitherto constant. The number of particle of a distinct types j is denoted by N j in (5.1), where j = 1,...,α. The respective intensive variable, (and respectively the j ), is denoted the chemical potentialThe chemical potential become identical to the
Fermi energy
for a gas of Fermions (at low temperatures, as we will discuss in Sect.13.3). Note that diffusion processes (e.g.
across a membrane) may change individual N j . The number of particles needs therefore not to be constant.Internal energy of a gas.
Eq. (5.1) is equivalent to
dU TdS PdVµdN ,
U U S,V,N )(5.2) 4748CHAPTER 5. THERMODYNAMIC POTENTIALS
for a gas with one species of particle, which implies that T �∂US�
V,N P =�∂UV�
S,N =�∂UN�
S,V (5.3)Response functions.
The experimentally important
response functions are obtained by second-order di ff erentiation of the internal energy, 2 U S 2 V,N =�∂TS�
V,N =T C V ,C VT�∂S
T�
V,NT�
2 U S 2 V,N 1 2 U V 2 S,N -�∂PV�
S,N =1 V S S =1V�
2 U V 2 S,N 1Maxwell relations.
AMaxwell relation
follows, as discussed already in Sect.4.4.2, from the di ff erentiability of thermodynamic potentials. An example of a Maxwell relation derived from the di ff erential of the internal energy U U S,V,N ) isV�
U∂N�
=∂∂N�U∂V�
V�
S -�∂PN�
V (5.4) which relates the change of the chemical potential with the volume V to the (negative of the) change of the pressure P with the number of particles N5.1.1 Monoatomic ideal gas
For the monoatomic ideal we did
fi nd hitherto PV nRT, U 32nRT, C
V =3 2nR . We now use these relations to derive an expression for U in terms of its natural variables.Entropy.
We start with the entropy
S T,V S T 0 ,V 0 ) =3nR2log�TT
0 nR log�V V 0 3 nR2log�UU
0 nR log�V V 0 of the ideal gas, as derived in Sect.4.4.1, where we have used the ideal gas relation
U = 3 nRT/2 to substitute
T and T 0 with U and U 0 in the second step.5.1. INTERNAL ENERGY U49
With C V = 3 nR/2 we may write equivalently
S S 0 C V = ln�U U 0 +23ln�VV
0 = ln� U U 0 �V V 0 2 3 (5.5) which can be solved for the internal energy as U S,V U 0 �V 0V�
1 e S S 0 /C V = 5 3 (5.6) Eq. (5.6) is the fundamental equation for the ideal gas, with U(S,V ) as the thermodynamic
potential. S,V are the independent natural variables.5.1.2 Thermodynamic potential vs. equation of state
The natural variables for
U are S and V , which means that if the function U S,V ) is known for a given system we can obtain -all- thermodynamic properties of the system through the di ff erentiation of U S,V ). The equation of state U U T,V,N )(5.7) for the internal energy U is on the contrary -not- a thermodynamic potential . This is because the fi rst derivatives of (5.7) yield the specific heat C
V and the energy equation4.12),�∂U
T�
V C V ,�∂UV�
TT�∂P
T�
V P , but not the dependent variables S and P . Note the marked di ff erence to ( 5.3).5.1.3 Classical mechanics vs. thermodynamics
There is a certain analogy between the potential
V and internal energy U of classical mechanics and thermodynamics respectively:Classical mechanicsThermodynamics
PotentialV (x,y,z)U(S,V,N)
Independent variablesx,y,zS,V,N
quotesdbs_dbs29.pdfusesText_35[PDF] La fonction logarithme népérien - Académie en ligne
[PDF] Fonction carré - Free
[PDF] Fonction inverse - Free
[PDF] Fonctions 1-def
[PDF] Les ensembles de nombres
[PDF] Méthodes sur le produit scalaire
[PDF] Intégration et théorie de la mesure
[PDF] 1 Ouvert, fermé, compact
[PDF] 1 Ouvert, fermé, compact
[PDF] Exercice 4 (fiche 2) Etablir si les ensembles sont ouverts, fermés
[PDF] Ouverts et fermés
[PDF] Logique, ensembles et applications - Exo7 - Emathfr
[PDF] enseñar geografía para los nuevos tiempos - Revistas UPEL
[PDF] calendrier des concours MINESUPpdf
