 Jasperse Acid-Base Chemistry. Extra Practice Problems
Jasperse Acid-Base Chemistry. Extra Practice Problems
A 0.100 M solution of a monoprotic weak acid has a pH of 3.00. What is the pKa of this acid? a. 5.00 d. 9.99 b. 0.999 e. 6.00.
 Acid-Base Titration and pH
Acid-Base Titration and pH
PROBLEMS Write the answer on the line to the left. Show all your work in the space provided. 4. [H3O ] in an aqueous solution 2.3 10 3 M. 4.3
 Exercise 14.3 - pH and pOH - Answers.pdf
Exercise 14.3 - pH and pOH - Answers.pdf
This value is called the ion product of water (Kw). The pH scale typically runs from 0-14 but very strong acids may have negative pH's
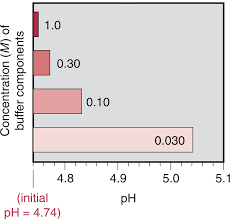 Section 19.1. Acid-Base Buffer Solutions
Section 19.1. Acid-Base Buffer Solutions
solution. (B) Calculate new pH (or pOH …) after something is added. Example: Sample Problem 19.1. (1) Calculate pH of a solution that is 0.50 M HAc and 0.50 M ...
 Exercise 15.4 - Titrations - Answers.pdf
Exercise 15.4 - Titrations - Answers.pdf
Solving Titration Problems. A titration is a chemical process for finding the The pH of any strong acid Istrong base titration at the equivalence point.
 Solutions to Review Problems for Acid/Base Chemistry
Solutions to Review Problems for Acid/Base Chemistry
1) are further diluted to exactly 800. mL the solution pH is 2.74. Calculate Ka for acetic acid. First
 Stanley Acids and Bases Study Guide
Stanley Acids and Bases Study Guide
pH and the pH scale and pH calculations. 15 – 20. 3.10 Titrations and This may assist especially when answering multiple choice questions. Base 1. Acid 1.
 1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
1 General Chemistry II Jasperse Buffers/Titrations/Solubility. Extra
Explain how a buffer solution manages to stabilize the pH against the addition of acid base
 1 Graphical Solution of Acid/Base Problems Graphical Solution of
1 Graphical Solution of Acid/Base Problems Graphical Solution of
• Stronger bases become protonated at higher pH than weaker bases. All available exchangeable. H+ ions are used to protonate available bases
 [PDF] Test2 ch17a Acid-Base Practice Problems
[PDF] Test2 ch17a Acid-Base Practice Problems
p3 Recognizing Acid/Base Properties when Ionics are Dissolved in Water p11 pH Calculations; Relationships between pH and pOH p4 Answers
 [PDF] Solutions to Review Problems for Acid/Base Chemistry
[PDF] Solutions to Review Problems for Acid/Base Chemistry
1) are further diluted to exactly 800 mL the solution pH is 2 74 Calculate Ka for acetic acid First there is a dilution followed by an equilibrium
 [PDF] Calculating pH and pOH worksheet Everett Community College
[PDF] Calculating pH and pOH worksheet Everett Community College
What is the pH of a 0 0235 M HCl solution? 2) What is the pOH of a Since there is both acid and base we will assume a 1 mole acid:1 mole base ratio of
 [PDF] Chapter 11 – Acids and Bases – Practice Problems Section 111
[PDF] Chapter 11 – Acids and Bases – Practice Problems Section 111
An Arrhenius acid produces H+ and an Arrhenius base produces OH- in aqueous solutions If you know the [OH-] how can you determine the pH of a solution?
 [PDF] Acid and Base pH Calculations – Supplemental Worksheet KEY
[PDF] Acid and Base pH Calculations – Supplemental Worksheet KEY
We will calculate the pH of the solutions using the following 3 steps for each problem Step 1: What is left in solution? Step 2: What are the equilibrium
 [PDF] Calculate the pH of the solution that results when 400 mL
[PDF] Calculate the pH of the solution that results when 400 mL
Worksheet: Acid base problems - AP level Problems 1 - 10 Problem #1: Calculate the pH of the solution that results when 40 0 mL of 0 100 M
 [PDF] Keys Acid Base Ch 15 pdf
[PDF] Keys Acid Base Ch 15 pdf
A solution of acid has [H] = 3 0 x 10³ M pH = 5 $47 pH = 8 597 7 A solution of base has an [OH] = 4 25 x 10 M Solve the problems below
 [PDF] 19_A_B_C_Worksheet_Keypdf - Humble ISD
[PDF] 19_A_B_C_Worksheet_Keypdf - Humble ISD
An acidic solution has a pH 7 0 BASE d pOH = 1 4 12 6 BASE 6 Classify the solutions in Problem 5 as acidic or basic
 [PDF] ACID – BASE EQUILIBRIUM
[PDF] ACID – BASE EQUILIBRIUM
3- Calculate the pH of the solution of all sorts of acids bases and A typical strong acid problem might be: What is the pH of a C a M HCl solution?
 [PDF] Acid-Base Titration and pH - David Brearley High School
[PDF] Acid-Base Titration and pH - David Brearley High School
The [H3O ] is 4 6 10 8 M in a solution Calculate the [OH ] PROBLEMS Write the answer on the line to the left Show all your work in the space provided
[PDF] acid base reaction problems with answers
[PDF] acid base titration problems with answers
[PDF] acid base titration problems with answers pdf
[PDF] acid base test review answers
[PDF] acid/base stoichiometry practice problems answers
[PDF] acide acétique
[PDF] acide base ph cours
[PDF] acide base ph exercice
[PDF] acide base ph terminale s
[PDF] acide base physique chimie
[PDF] acide base physique terminale s
[PDF] acide base physique ts
[PDF] acide et base conjuguée
[PDF] acide et base de bronsted
