 Kinetic modeling of homogeneous catalytic processes
Kinetic modeling of homogeneous catalytic processes
Nov 14 2013 The mechanism of homogeneous catalytic reactions is complex even if only a single reaction is involved
 The Cativa Process for the Manufacture of Acetic Acid
The Cativa Process for the Manufacture of Acetic Acid
In 1996 a new process for the carbonylation of methanol to acetic acid was announced by BP. Chemicals based on a promoted iridium catalyst package
 Kinetic Study Modeling and Simulation of Homogeneous Rhodium
Kinetic Study Modeling and Simulation of Homogeneous Rhodium
through water gas shift reaction. 3. 3. CH OH CO CH COOH. +. →. (1). Based on the Monsanto process Celanese Corporation and Daicel Chemical Industries used
 Sustainable routes for acetic acid production: Traditional processes
Sustainable routes for acetic acid production: Traditional processes
May 9 2022 However
 Stepwise Iodide-Free Methanol Carbonylation via Methyl Acetate
Stepwise Iodide-Free Methanol Carbonylation via Methyl Acetate
reaction as a side-reaction during the Cativa process.13
 Co-production of Acetic Acid and Hydrogen/Power from Natural Gas
Co-production of Acetic Acid and Hydrogen/Power from Natural Gas
using iridium: the Cativa™ process for the manufacture of acetic acid. Reaction over a Ni‐Al2O3 Catalyst‐Catalyst Stability Reaction Kinetics and Reactor.
 Promotion of Iridium-Catalyzed Methanol Carbonylation
Promotion of Iridium-Catalyzed Methanol Carbonylation
The Cativa Process. The key features of the Cativa process have been insertion reaction (as demonstrated by the model kinetic studies). These species can ...
 CHEMICAL ENGINEERING DESIGN Principles Practice and
CHEMICAL ENGINEERING DESIGN Principles Practice and
He manages the areas of process design and optimization equipment design
 Enzymatic conversion of carbon dioxide
Enzymatic conversion of carbon dioxide
May 12 2015 synthetic processes
 Kinetic modeling of homogeneous catalytic processes
Kinetic modeling of homogeneous catalytic processes
Kinetic modeling of homogeneous catalytic processes. R.V. Chaudhari. ?. A. Seayad
 Kinetic Study Modeling and Simulation of Homogeneous Rhodium
Kinetic Study Modeling and Simulation of Homogeneous Rhodium
manufacturing of acetic acid is homogeneous methanol carbonylation through the chemical Eq. (1). The Monsanto process (Rh: catalyst; CH3I: promoter;
 Co-production of Acetic Acid and Hydrogen/Power from Natural Gas
Co-production of Acetic Acid and Hydrogen/Power from Natural Gas
The iridium-based CATIVA process offers significant advantages over the earlier kinetics of the reaction including the reported advantages of this ...
 On-Purpose Acetic Acid
On-Purpose Acetic Acid
1 ago 2016 The BP Cativa™ process for production of acetic acid by carbonylation ... to productivity and kinetics with low water in the Chiyoda process.
 Process Simulation for the Design and Scale Up of Heterogeneous
Process Simulation for the Design and Scale Up of Heterogeneous
18 may 2017 However the core of the sizing of chemical reactors remains the availability of a detailed kinetic model. Kinetics of heterogeneous catalytic ...
 Reactivity of Ir (iii) carbonyl complexes with water: alternative by
Reactivity of Ir (iii) carbonyl complexes with water: alternative by
17 sept 2013 kinetic experiments are consistent with a mechanism involving nucleophilic ... iodide catalysed methanol carbonylation process Cativa™
 Promotion of Iridium-Catalyzed Methanol Carbonylation
Promotion of Iridium-Catalyzed Methanol Carbonylation
methanol carbonylation process Cativa
 Review - The Chemistry of CO: Carbonylation
Review - The Chemistry of CO: Carbonylation
14 mar 2019 33A).102 Radical-trapping and kinetic isotope experiments ... Monsanto and Cativa processes have already been industrialized and serve.
 Techno-Economic Evaluation of Novel Hybrid Biomass and
Techno-Economic Evaluation of Novel Hybrid Biomass and
18 feb 2022 the kinetic model by Vanden Bussche and Froment (1996) with ... Later
Catalysis Today 66 (2001) 371-380
Kinetic modeling of homogeneous catalytic processesR.V. Chaudhari
, A. Seayad, S. JayasreeHomogeneous Catalysis Division, National Chemical Laboratory, Dr. Homi Bhabha Road, Pune 411 008, India
Abstract
Homogeneous catalysis by soluble metal complexes is gaining considerable attention due to their unique applications and
features like high activity and selectivity. In this paper, a brief review of kinetic modeling in homogeneous catalysis has been
presented. Approaches using empirical as well as molecular level rate models have been discussed. Special features relevant
of 1-(4-iso-butylphenyl)ethanol using a homogeneous palladium catalyst has been discussed. © 2001 Elsevier Science B.V.
All rights reserved.Keywords:Homogeneous catalysis; Kinetics; Rate equation; Hydrogenation; Hydroformylation; Carbonylation; 1-(4-iso-Butylphenyl)ethanol;
Ibuprofen
1. Introduction
Kinetic modeling of catalytic reactions is one of the key aspects investigated in order to understand the rate behavior of catalytic reactions [1-10] as well as reaction mechanism [5-7]. A knowledge of intrinsic reaction kinetics (a scale independent property) and development of rate equations is most essential as a part of reaction engineering studies aimed to evolve strategy for reactor design. While, the subject of kine- tic modeling has been well investigated for heteroge- neous catalysis [8-10], only limited information is available on this aspect in homogeneous catalysis [1-7].Homogeneous catalysts consisting of soluble tran-
sition metal complexes have several important ap- plications in chemical industry for both bulk com- modity as well as specialty products [11-15]. Some important examples are listed in Table 1. The newly?Corresponding author. Tel.:+91-20-589-3163;
fax:+91-20-589-3260. E-mail address:rvc@ems.ncl.res.in (R.V. Chaudhari). emerging applications in fine chemicals and pharma- ceuticals are particularly promising due to increased competition along with a need for selective, effi- cient and environmentally acceptable processes. An- other important feature is their high selectivity for the synthesis of biologically active molecules with asymmetric centers [16]. Since, most of the new drug molecules are expected to be optically active isomers, homogeneous catalysis has a bright future in pharmaceutical industry. Homogeneous catalysis has so far been investigated with the perspective of reaction mechanism, in which the role of catalysts, ligands, co-catalysts and nature of catalytically active species have been studied [11-15]. While, a num- ber of examples illustrate systematic studies of in situ spectroscopic analysis of catalytic reaction in- termediates leading to description of catalytic cycles on a molecular level [17-19], correlation of these with kinetic data and development of rate equations has received limited attention. In this paper, the cur- rent state of development on kinetic modeling in homogeneous catalysis has been presented with aspecific case study on kinetics of carbonylation of0920-5861/01/$ - see front matter © 2001 Elsevier Science B.V. All rights reserved.
PII:S0920-5861(00)00633-7
372R.V. Chaudhari et al./Catalysis Today 66 (2001) 371-380
Nomenclature
B l concentration of IBPE in the liquid phase at timet B 0 initial concentration of IBPE in the liquid phase D l concentration of IBS in the liquid phase at timet E l concentration of IBPCl in the liquid phase at timet k i rate constants k -1min mac dissociation rate constant for the minor diastereomer k 1maj mac binding rate constant for the major diastereomer k 1min mac binding rate constant for the minor diastereomer k 2maj rate constant for the H 2 addition step forthe major diastereomer k 2min rate constant for the H 2 addition step for the minor diastereomer K i equilibrium or empirical constants K 1maj mac binding equilibrium constant for the major diastereomer K 1min mac binding equilibrium constant for the minor diastereomer m,n,preaction orders as given in Eq. (6) P CO partial pressure of CO P C 2 H 4 partial pressure of C 2 H 4 P l concentration of carbonylated products(IBN+IPPA)in the liquid phase at timet r i rate of the reaction r iso rate of formation of theiso-isomer r n rate of formation of then-isomer rR-product
rate of formation of theR-product rS-product
rate of formation of theS-product [X] concentration of the species X1-(4-iso-butylphenyl)ethanol (IBPE) using homo-
geneous Pd complex catalyst.2. Kinetic models in homogeneous catalysis
As a first step in kinetic modeling, it is important to consider the reaction pathways and catalytic reaction mechanism for any given system. The mechanism ofhomogeneous catalytic reactions is complex even if only a single reaction is involved, since the catalytic cycle consists of several stoichiometric reactions. When a co-catalyst or a promoter is used, additional steps are associated with the catalytic cycle either to form the active catalytic species around which the principal catalytic cycle operates or to form an active substrate. The reactions may also involve one or more gas phase reactants or biphasic systems with catalyst and reactants/products present in different phases. These multi-phase catalytic gas-liquid reactions need consideration of interphase mass transfer steps in addition to the overall catalytic reactions. Thus, ho- mogeneous catalytic reactions can be categorized as follows:1. Single or multi-step reactions with only one cata-
lytic component: the examples of this class are found in hydrogenation of olefins using RhCl- (PPh 3 3 , hydroformylation of olefins usingHRh(CO)(PPh
3 3 , oligomerization of ethylene using Ni complex catalyst, etc.2. Singleormulti-stepreactionswithmulti-component
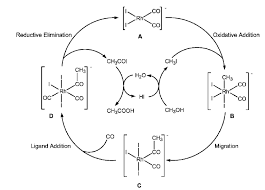
catalyst systems (catalyst/co-catalyst/promoter): the examples of this category include the Wacker process for oxidation of ethylene to acetaldehyde using PdCl 2 /CuCl 2 and molecular oxygen. During conversion of ethylene to acetaldehyde, Pd 2+ is reduced to Pd 0 and the co-catalyst CuCl 2 has a role to re-oxidize Pd 0 to the active Pd 2+ [20]. Also the molecular oxygen has a role to re-oxidize the reduced co-catalyst and hence is only indirectly involved in catalysis. The re-oxidation is required to be faster than main oxidation reaction for the catalytic cycle to operate efficiently. Another im- portant example is the carbonylation of methanol to acetic acid using Rh complex with HI as a pro- moter. In this case, the promoter HI converts the substrate methanol to CH 3I, an active substrate
for carbonylation and the catalytic reaction pro-quotesdbs_dbs21.pdfusesText_27[PDF] cats are nice
[PDF] cats as pets
[PDF] cauchy sequence example
[PDF] cauchy sequence pdf
[PDF] cause and effect essay
[PDF] cause and effect essay outline
[PDF] cause and effect essay pdf
[PDF] cause and effect paragraph
[PDF] cause and effect paragraph pdf
[PDF] cause de mortalité dans le monde
[PDF] cause de mortalité dans le monde 2020
[PDF] cause de mortalité dans le monde animal
[PDF] cause de mortalité dans le monde oms
[PDF] cause no d 1 gn 15 004985
