 Kinetic modeling of homogeneous catalytic processes
Kinetic modeling of homogeneous catalytic processes
Nov 14 2013 The mechanism of homogeneous catalytic reactions is complex even if only a single reaction is involved
 The Cativa Process for the Manufacture of Acetic Acid
The Cativa Process for the Manufacture of Acetic Acid
In 1996 a new process for the carbonylation of methanol to acetic acid was announced by BP. Chemicals based on a promoted iridium catalyst package
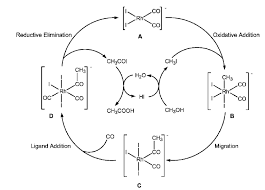 Kinetic Study Modeling and Simulation of Homogeneous Rhodium
Kinetic Study Modeling and Simulation of Homogeneous Rhodium
through water gas shift reaction. 3. 3. CH OH CO CH COOH. +. →. (1). Based on the Monsanto process Celanese Corporation and Daicel Chemical Industries used
 Sustainable routes for acetic acid production: Traditional processes
Sustainable routes for acetic acid production: Traditional processes
May 9 2022 However
 Stepwise Iodide-Free Methanol Carbonylation via Methyl Acetate
Stepwise Iodide-Free Methanol Carbonylation via Methyl Acetate
reaction as a side-reaction during the Cativa process.13
 Co-production of Acetic Acid and Hydrogen/Power from Natural Gas
Co-production of Acetic Acid and Hydrogen/Power from Natural Gas
using iridium: the Cativa™ process for the manufacture of acetic acid. Reaction over a Ni‐Al2O3 Catalyst‐Catalyst Stability Reaction Kinetics and Reactor.
 Promotion of Iridium-Catalyzed Methanol Carbonylation
Promotion of Iridium-Catalyzed Methanol Carbonylation
The Cativa Process. The key features of the Cativa process have been insertion reaction (as demonstrated by the model kinetic studies). These species can ...
 CHEMICAL ENGINEERING DESIGN Principles Practice and
CHEMICAL ENGINEERING DESIGN Principles Practice and
He manages the areas of process design and optimization equipment design
 Enzymatic conversion of carbon dioxide
Enzymatic conversion of carbon dioxide
May 12 2015 synthetic processes
 Kinetic modeling of homogeneous catalytic processes
Kinetic modeling of homogeneous catalytic processes
Kinetic modeling of homogeneous catalytic processes. R.V. Chaudhari. ?. A. Seayad
 Kinetic Study Modeling and Simulation of Homogeneous Rhodium
Kinetic Study Modeling and Simulation of Homogeneous Rhodium
manufacturing of acetic acid is homogeneous methanol carbonylation through the chemical Eq. (1). The Monsanto process (Rh: catalyst; CH3I: promoter;
 Co-production of Acetic Acid and Hydrogen/Power from Natural Gas
Co-production of Acetic Acid and Hydrogen/Power from Natural Gas
The iridium-based CATIVA process offers significant advantages over the earlier kinetics of the reaction including the reported advantages of this ...
 On-Purpose Acetic Acid
On-Purpose Acetic Acid
1 ago 2016 The BP Cativa™ process for production of acetic acid by carbonylation ... to productivity and kinetics with low water in the Chiyoda process.
 Process Simulation for the Design and Scale Up of Heterogeneous
Process Simulation for the Design and Scale Up of Heterogeneous
18 may 2017 However the core of the sizing of chemical reactors remains the availability of a detailed kinetic model. Kinetics of heterogeneous catalytic ...
 Reactivity of Ir (iii) carbonyl complexes with water: alternative by
Reactivity of Ir (iii) carbonyl complexes with water: alternative by
17 sept 2013 kinetic experiments are consistent with a mechanism involving nucleophilic ... iodide catalysed methanol carbonylation process Cativa™
 Promotion of Iridium-Catalyzed Methanol Carbonylation
Promotion of Iridium-Catalyzed Methanol Carbonylation
methanol carbonylation process Cativa
 Review - The Chemistry of CO: Carbonylation
Review - The Chemistry of CO: Carbonylation
14 mar 2019 33A).102 Radical-trapping and kinetic isotope experiments ... Monsanto and Cativa processes have already been industrialized and serve.
 Techno-Economic Evaluation of Novel Hybrid Biomass and
Techno-Economic Evaluation of Novel Hybrid Biomass and
18 feb 2022 the kinetic model by Vanden Bussche and Froment (1996) with ... Later
Gotthard Seifert
b,? , Jean-SabinMcEwen a,c,d,e,? , YongWang a,e,? aThe Geneand LindaVoiland Schoolof ChemicalEngineering andBioengineering, WashingtonState University,Pullman, WA99164, UnitedStates
b c Department ofPhysics andAstronomy, WashingtonState University,Pullman, WA99164, UnitedStates d Department ofChemistry, WashingtonState University,Pullman, WA99164, UnitedStates eInstitute forIntegrated Catalysis,Pacific NorthwestNational Laboratory,Richland, WA99352, UnitedStates
articleinfoArticle history:
Received 17July 2017
Revised 21February 2018
Accepted 22February 2018
Available online5 April2018
Keywords:
Single-site heterogeneouscatalyst
Methanol carbonylation
IrALa complex
Promoter effects
Density functionaltheory
Attenuated totalreflectance-Fourier
transform infraredspectroscopy abstract The creationof heterogeneousanalogs tohomogeneous catalystsis ofgreat importance tomany indus-trial processes.Acetic acidsynthesis viathe carbonylationof methanolis onesuch processand itrelies on
a difficult-to-separatehomogeneou sIr-basedcatalyst.Using acombination ofdensity functionaltheory (DFT) andattenuated totalreflect ance-Fouriertransform infrared(ATR-FTIR)spectroscopy,we determine the structureand mechanismfor methanolcarbonylation overa promisingsingle-site IrALa/C heteroge- neous catalystreplacement. Here,the Ircenter isthe activesite withthe acetyl-Ircomplex beinga rate controllingintermediat e.Furthermore,theLa bothatom icallydisperses theIr andacts asaLewisacid site. Infa ct,theLapromoter inthe IrALa/C catalystwas foundto behavesimilarly tohomogeneous pro- moters byabstracting aniodine fromthe Ircenter andaccelerating theCO insertionstep. Overall,this work provideskey insight intotheatomisticnature ofthe IrALa/C single-sitecatalyst andallows for the furtherdesign andoptimization ofsingle-site heterogeneouscatalysts. ?2018 ElsevierInc. Allrights reserved.1. Introduction
Single-site heterogeneouscatalysts area newclass ofmaterials that interfacethe heterogeneousand homogeneous paradigms, combining thehigh activityand selectivityof homogeneous cata- lysts withthe highseparability ofheterogeneous catalysts.Exam- ples ofsuch catalystsinclude bimetallic surfaceswith an atomically dispersednoble metalsupported ona basemetal or oxide surface,as wellas beingexchanged intozeolites [1-9]. One industrially homogeneouscatalytic processcurrently withouta viable heterogeneousreplacement [10-12]is theproduction of acetic acidwhich isaccomplis hedvia thecarbonylationof metha- nol througheither theMonsant oprocess withanRhcatalyst [10,13,14]or theCativa processwith anIr catalystwith aRu pro- moter[14-18]. Themechanism forthe catalysishas thesamemajor stepsfor bothprocesses [14,15], withthe keysteps beingthe oxidativeaddition ofiodomethane (MeI)to thereduced metalcenter ([M(CO)2
I 2 ) andthe migratoryinsertion ofCO intothe methyl-metalbond followedby thereductive eliminationof acetyl iodide (AcI)as theproduct [10,19-21]. Inour recentwork, we reported apromising heterogeneouscatalyst forthe carbonylation of methanolbased onIr anda Lapromoter [11]. Characterizationof this IrALa/C systemshowed thatthe catalystwas amolecular spe- cies withdistinct sitesformed fromtwo metalatoms [11], essen- tially creatinga single-sitecatalyst onan activatedcarbon support. Overall,the heterogeneous IrALa/C catalystshowed com- parable reactivityand selectivityto theanalogous homogeneous catalyst[11]. Furthermore,using Laas apromoter asopposed to Ru canalso reducethe costof thecatalyst. Thus,the IrALa/C heterogeneouscatalyst isa promisingalternative tothe conven- tional homogeneoussystem forthe carbonylationof methanolto acetic acid. Here, weestablish thestructure andmethanol carbonylation mechanismof theheterogeneous IrALa/C catalystusing acombi- nation ofdensity functionaltheory (DFT)and attenuatedtotal reflectance-Fouriertransform infrared(ATR-FTIR) spectroscopy. Additionally,we haveelucidate dthe roleoftheLa promoterin the carbonylationreaction. Overall,this workshows thatthe https://doi.org/10.1016/j.jcat.2018.02.0220021-9517/?2018 ElsevierInc. Allrights reserved.
Corresponding authorsat: TheGene andLinda VoilandSchool ofChemical Engineering andBioengineering, WashingtonState University,Pullman, WA99164,United States(J.-S. McEwenand Y.Wang).
E-mail addresses:gotthard.seifert@chemie.tu-dresden.de(G. Seifert), js.mcewen@wsu.edu(J.-S. McEwen),yong.wang@pnnl.gov(Y. Wang).Journal ofCatalysis 361(2018) 414-422
Contents listsavailable atScienceDirect
Journalof Catalysis
journal homepage:www.else vier.com/locate/jcat dispersion andactivity ofsingle-site heterogeneouscatalysts can be tunedvia thechoice ofpromoter (i.e.altering thepromoter"s oxygen affinityand electronegativity/Lewis acidity),thereby allowing forthe greaterdesign andoptimization ofheterogeneous catalysts forthe carbonylationof methanol.2. Methodsand materials
2.1. Densityfunctional theory
DFT calculationswerecarried outusing theVienna Ab Initio Simulation Package(VASP) [22-24]. Theprojector-augmen ted wave (PAW)method [25,26]with aplane-wave basisset and an energycutoff of450 eVwere used.To modelthe electron exchange andcorrelation, thePerdew-Burke-Er nzerhof(PBE) functional[27]has beenapplied. Spinpolarization hasbeen included inall calculations. TheGaussiansmearing[28]method was usedwith asmearing widthof 0.2eV toimprove conver- gence, andthe totalenergy wasextrapolated tozero Kelvin.All gas phaseground stateoptimizati onsused theconjugategradi- ent methodand wereconsidered converged whenthe inter- atomic forceswere smallerthan 0.01eV/Å, whilesurface relaxations wereconsidered convergedwhen theforces wereless than 0.025eV/Å. Theenergy tolerancewas setto 10 ?7 eV. Calcu- lations formolecules inthe gasphase wereperformed usingan18?19?20 Åbox, andone singlek-point, theGamma point,
was sufficientto spanthe Brillouinzone. Ab InitioMolecular Dynamics (AIMD)simulations havebeen computedfor aNVT ensemble at513 K,which isthe reactiontemperature. Asthe spin-polarizedground stateoptimizations resultedin anet zero magnetic momentfor thecomplexes examined here,the AIMD calculations werenot spinpolarized andthe energytolerance was setto 10 ?5 eV. Thetransition statescalculated herewere obtained usingthe ClimbingImage NudgedElastic Band(CINEB) method[29]. Theoptimizations alongthe minimumenergy path- ways (MEPs)were performedwith thefast inertialrelaxation engine (FIRE)optimizer withforce andenergy tolerancesof0.05 eV/Åand 10
?7 eV, respectively[30,31]. Eachtransition state was foundto haveone imaginaryvibrational modealong agiven reaction pathway[32]. In orderto makea strongerconnection betweenthe theoryand experiment inthis work,we calculatedthe Gibbsenergy forour tested methanolcarbonylationreaction pathwaysusing standard statistical mechanicsprinciples [33]. TheGibbs energyfor each configurationwas calculatedas: G i ¼E i ?k B Tln q trans q rot q vibðÞð1Þ
whereE i is theDFT-calculated energyfor theith configuration; q trans ,q rot , andq vib are thetranslation, rotational,and vibrational partition functions;and k B andTare Boltzmann"sconstant and the reactiontemperature ,respectively.In thisstudy,thegas phase IrALa complexeswere treatedas themodel surfacein eachcase, and thereforeonly thevibrational entropyassociated witheach complex wasincluded inthe Gibbsenergies. However,the transla- tional, rotational,andvibrational entropycontributions fromthe gas phaseCO, MeI,and AcIspecies wereincluded forthe relevant ground stateconfigura tions.Thepartitionfunctions werecalcu- lated accordingto [34]: q trans 2pmk BTðÞ
3=2 h 3 k B TPð2Þ
q rot;linear 8p 2 I linear k B T h 2ð3Þq
rot;non?linear 8p 2 2pk BTðÞ
3=2 ffiffiffiffiffiffiffiffiffiffiffiffiI A I B I C p rh 3ð4Þ
q vib ¼Y i e ?ht i =2k B T 1?e ?ht i =k B Tð5Þ
wheremandPare themass andpartial pressureof themole- cule ofinterest; I linear is themoment ofinertia fora linearmolecule; I A ,I B , andI C are theprincipal momentsof inertiaof themolecule of interest (non-linearcase); ris thesymmetry numberfor themole- cule ofinterest; and m i is theith vibrational mode.AllGibbsener- gies werecalculated ata temperatureof 513K, atotal pressureof17 bar,a COconcentration of17.3 mol%,a MeIconcentration of1.8
mol%, andan AcIconcentratio nof 1.8mol%,consistentwith the catalytic experimentsperformedin ourprevious work(see Supple- mentary Materialfor moredetails) [11]. For thesurface calculations, asinglelayercarbon sheetwas simulated torepresent theactivated carbonsupport. Thesingle layer sheetwas foundto besufficient asonly weakinteractions exist betweendifferent layersof graphiteand theinfluence ofmul- tiple graphenelayers onadsorptio nenergies isnegligible[35]. Two different adsorptionsites havebeen modeledusing VASPand are shown inFig. S1;a pristinegraphene sheetto representthe basal planes andan OH-passivated armchair-graphenenanoribon (AGNR-OH)to simulatean adsorptionedge (Fig.S1). Anoptimized lattice constantof 2.467Å wasfound forgraphene whichis consis-quotesdbs_dbs21.pdfusesText_27[PDF] cats are nice
[PDF] cats as pets
[PDF] cauchy sequence example
[PDF] cauchy sequence pdf
[PDF] cause and effect essay
[PDF] cause and effect essay outline
[PDF] cause and effect essay pdf
[PDF] cause and effect paragraph
[PDF] cause and effect paragraph pdf
[PDF] cause de mortalité dans le monde
[PDF] cause de mortalité dans le monde 2020
[PDF] cause de mortalité dans le monde animal
[PDF] cause de mortalité dans le monde oms
[PDF] cause no d 1 gn 15 004985
