 Solutions
Solutions
Chemistry the partial vapour pressure of each component of the solution is Another important class of solutions consists of solids dissolved in liquid ...
 D:TEXTBOOKSRATIONALISED 20222-23NehaP85
D:TEXTBOOKSRATIONALISED 20222-23NehaP85
V. 0.130. = 3.17 V – 0.21V = 2.96 V. Solution. Rationalised 2023-24. Page 10. 40. Chemistry.
 Section 4.1: Types of Chemical Bonds
Section 4.1: Types of Chemical Bonds
Page 12. Copyright © 2012 Nelson Education Ltd. Chapter 4: Chemical Bonding 7. Answers will vary. Sample answer: Coordinate covalent bonding is like you ...
 Chem 12 SM Ch5 Review final new ok revised
Chem 12 SM Ch5 Review final new ok revised
Δ = −. Statement: The enthalpy change ΔH
 Chem12 SM Ch05 Section5 3 final ok revised
Chem12 SM Ch05 Section5 3 final ok revised
Section 5.3 Questions page 313. 1. (a) Solution: Step 1: Use the balanced chemical equation to determine the bonding of each substance. H2(g) +
 Chem12 SM Ch05 Section5 2 final ok revised
Chem12 SM Ch05 Section5 2 final ok revised
Tutorial 3 Practice page 304. 1. (a) Solution: Step 1: Write the balanced chemical equation without the energy term. 2 C2H2(g) + 5 O2
 Grade 12 Chemistry: A Foundation for Implementation
Grade 12 Chemistry: A Foundation for Implementation
Jan 21 2011 ... textbook. The successful implementation of Grade 12 Chemistry depends ... solutions based on this result? Explain. 3. Are there any reactions ...
 Chem12 SM Ch7 Section7.5 final ok revised
Chem12 SM Ch7 Section7.5 final ok revised
Solution: Step 1. Convert the given initial amount of cyclopropane gas to (c) The chemical system is not at equilibrium. We have been given K = 1.2 × 102 ...
 Section 8.4: Calculations Involving Acidic Solutions
Section 8.4: Calculations Involving Acidic Solutions
Section 8.4: Calculations Involving Acidic Solutions. Tutorial 1 Practice page x2 ≈ 6.2 × 10−12 x = [H+(aq)]. ≈ 2.49 × 10−6 mol/L. pH = −log(2.49 × 10 ...
 Chem12 SM Ch8 Section8e1 final ok revised
Chem12 SM Ch8 Section8e1 final ok revised
solution; an Arrhenius base forms hydroxide ions in aqueous solution. (b) A Brønsted–Lowry acid is a proton donor; a Brønsted–Lowry base is a proton.
 Grade 12 Chemistry: A Foundation for Implementation
Grade 12 Chemistry: A Foundation for Implementation
Jan 21 2011 promotes the idea that all answers are enshrined in a textbook. The successful implementation of Grade 12 Chemistry depends on a ...
 Chem12 SM Ch7 Section7.6 final revised
Chem12 SM Ch7 Section7.6 final revised
Chapter 7: Chemical Equilibrium 7.6-1 Write a balanced equation for the solution equilibrium. Ca3(PO4)2(s) ! "!# !! ... Ksp of CuI(s) = 1.3 × 10?12.
 Chem12 SM Ch05 Section5 2 final ok revised
Chem12 SM Ch05 Section5 2 final ok revised
Chapter 5: Thermochemistry. 5.2-3. Solution: Step 1: Determine the change in temperature AT . final initial. 27.8 °C 19.8 °C.
 Chem 12 SM Ch5 Review final new ok revised
Chem 12 SM Ch5 Review final new ok revised
(a) Answers may vary. Sample answer: Hydrogen gas has a high enthalpy of combustion releasing about 2.5 times the quantity of energy per gram than methane but
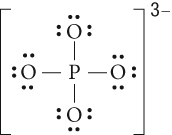
 Solutions
Solutions
Chemistry. Type of Solution. Solute. Solvent. Common Examples. Gaseous Solutions. Gas. Gas. Mixture of oxygen and nitrogen gases.
 Chem12 SM Ch7 Section7.5 final ok revised
Chem12 SM Ch7 Section7.5 final ok revised
Chapter 7: Chemical Equilibrium Solution: N2O4(g) ! "!# !! 2 NO2(g) ... Using the balanced chemical equation construct an ICE table for calculating.
 Chem12 SM Ch05 Section5.4 final ok revised
Chem12 SM Ch05 Section5.4 final ok revised
An enthalpy diagram of the reaction is: Page 2. Copyright © 2012 Nelson Education Ltd. Chapter 5: Thermochemistry. 5.4-2. 2. (a) Solution: Step 1: Label the
 chemistry grade 12 studen textbook
chemistry grade 12 studen textbook
CHEMISTRY GRADE 12. 2. MAIN CONTENTS. 1.1 Homogeneous and Heterogeneous Mixtures. 1.2 Types of Solutions. 1.3 The Solution Process.
 EngLinks
EngLinks
Grade 12 Chemistry Review Workbook. 1 of 22. 1. Organic Chemistry. General IUPAC rules for naming organic compounds: 1) The lowest numbers possible must be
 Chem12 SM Ch05 Section5 3 final ok revised
Chem12 SM Ch05 Section5 3 final ok revised
combustion of ethene gas to gaseous carbon dioxide and liquid water is an exothermic reaction. 3. Solution: Step 1: Use the balanced chemical equation:.
Grade 12 Chemistry
Review Workbook -
Solutions
Created by Kali Zender
Commented [KZ1]: Tutor: Include brief content
summaries but focus mainly on examples for time purposes. Sections which should be the first to be skipped or shortened are indicated (they are less important to cover).Grade 12 Chemistry Review Workbook
1 of 22
1. Organic Chemistry
General IUPAC rules for naming organic compounds:
1) The lowest numbers possible must be used to denote positions.
2) To separate numbers, use commas.
3) To separate numbers and letters, use hyphens.
4) Names of branches and parent chains are not to be separated.
5) Unnecessary numbers should be omitted.
The parent chain is the longest carbon chain of an organic compound. The length of the parent chain is
associated with a certain prefix, as shown below. Prefix Number of Carbons in Parent Chain Prefix Number of Carbons in Parent Chain meth- 1 hex- 6 eth- 2 hept- 7 prop- 3 oct- 8 but- 4 non- 9 pent- 5 dec- 101.1 Alkanes, Alkenes, and Alkynes
A hydrocarbon is an organic compound that contains only carbon and hydrogen atoms.Hydrocarbon Type Carbon-Carbon Bonds Saturation
Alkane Only single bonds Saturated
Alkene At least one double bond Unsaturated
Alkyne At least one triple bond Unsaturated
A saturated hydrocarbon ǡDzdz
number of hydrogen atoms. In contrast, unsaturated hydrocarbons contain double or triple bonds, allowing for less hydrogen atoms to be bonded to each carbon atom.1.1.1 Naming Branched Alkanes, Alkenes, and Alkynes
Straight chains become branched chains with the addition of alkyl groups, which are hydrocarbongroups derived from alkanes by the removal of an H atom, e.g. -CH3 γDzǡdz-C2H5 γDzǤdz Use the
steps below as a guide to name branched alkanes.1) Identify the parent chain.
2) Number the carbon atoms, starting with the end closest to the multiple bond, if present. If no
multiple bonds are present, start naming with the end closest to the branch(es).3) Indicate the location of the multiple bond, if present, by the number of the carbon atom that begins
the multiple bond. Indicate multiple doublDz-dzȋ͚ȌǡDz-dzȋ͛ȌǡDz-dz
(4), etc.4) Name each branch and indicate its location on the parent chain by the number of the carbon atom
at the point of attachment. If more than 1 branch is present, list the branches in alphabetical order.
If there are multiple identical branches, insert a comma between their location numbers and useGrade 12 Chemistry Review Workbook
2 of 22
5) Write the complete IUPAC name, including both multiple bonds and branches. Use Dz-dz
for alkanesǡDz-dzǡDz-dz.1.1 Example
Name the following organic compounds.
a)Answer: ethene
b)Answer: 6-methyl-2-octene
c)Answer: 3-methyl-1,4-hexadiyne
d)Answer: 2-methylpentane
1.2 Halogenoalkanes
Halogenoalkanes (also called haloalkanes or alkyl halides) are formed when halogen atoms (fluorine,chlorine, bromine, or iodine) are added to a parent chain to replace a hydrogen atom. Halogenoalkanes
have the general formula CnH2n+1X, where X denotes the halogen atom.1.2.1 Naming Halogenoalkanes
Halogenoalkanes are named by treating the halogens as branches. The halogen branch names are Dz-ǡdzDz-ǡdzDz-ǡdzDz-Ǥdze indicated by the suffixes1.2 Example
Name the following organic compounds.
1.1Answer: 1-chloro-2,2-dimethylpropane
1.2Answer: 1,3-dibromo-2-chlorobutane
1.3 Alcohols
Alcohols are organic compounds which include a hydroxyl functional group (-OH) and have a general formula CnH2n+1OH. Alcohols with the same formula may differ by the location of the hydroxyl group.1.3.1 Naming Alcohols
To indicate the hydroxyl functional group, aDz-dzȋDz-dz first). Numbering must start at the end nearest the hydroxyl group. The hydroxyl group position is indicated by the number associated with the carbon atom to which it is attached. This number can1.3 Example
Grade 12 Chemistry Review Workbook
3 of 22
Name the following organic compounds.
a)Answer: 1-propanol or propan-1-ol
b)Answer: 2-methyl-2-butanol or 2-
methylbutan-2-ol1.4 Ethers
Ethers are organic compounds containing 2 alkyl groups attached to an oxygen atom.1.4.1 Naming Ethers
Dz-dzplaced after the prefix of the smaller hydrocarbon group, and the alkane name of the larger hydrocarbon group is added at the end.1.4 Example
Name the following organic compounds.
a)Answer: methoxymethane
b)Answer: ethoxypropane
1.5 Aldehydes and Ketones
Both aldehydes and ketones contain a carbonyl functional group (C=O). Aldehydes and ketones differ by the position of their carbonyl groups, as shown below. Organic Compound Carbonyl Group Position General StructureAldehyde Dzdzǣa hydrogen atom
Ketone Bonded to 2 carbon atoms
1.5.1 Naming Aldehydes
Dz-dzDz-Ǥdz No numbering is necessary to indicate the position of the carbonyl group since it is always located at the end of the molecule.1.5.2 Naming Ketones
containing the carbonyl group has more than 4 carbon atoms, it is necessary to use a numerical prefix
to specify the location of the carbonyl group (if less than 4 carbons, the number would be redundant).
1.5 Example
Name the following organic compounds.
Grade 12 Chemistry Review Workbook
4 of 22
a)Answer: 2-pentanone
b)Answer: methanal
1.6 Carboxylic Acids and Esters
Both carboxylic acids and esters contain a carbonyl functional group with an additional oxygen atomsingle-bonded to the carbon atom. Carboxylic acids and esters differ by the position of their carbonyl
groups, as shown below. Organic Compound Carbonyl Group Position General Structure Carboxylic Acid DzdzǣThe added oxygen is bonded to a hydrogen atomEster The added oxygen is bonded to a carbon atom
1.6.1 Naming Carboxylic Acids
1.6.2 Naming Esters
Write the name of the alkyl group attached to the singly bonded oxygen on the carbonyl group. Follow with the name of the chain bonded to the other side of the carbonyl group, changing the suffix Dz-1.6 Example
Name the following organic compounds.
a)quotesdbs_dbs2.pdfusesText_2[PDF] chemistry notes for class 12 pdf
[PDF] chiffres coronavirus france 11 mai
[PDF] chiffres coronavirus france 11 mai 2020
[PDF] chiffres covid france 6 juin
[PDF] child care cost per province
[PDF] child language acquisition stages
[PDF] childhood in france vs us
[PDF] china paris agreement goals
[PDF] china population 2019 vs us
[PDF] chinese food near me open
[PDF] chinese language cantonese vs mandarin
[PDF] chinese restaurant in paris tx
[PDF] choix heure ete ou hiver france
[PDF] chômage technique code travail maroc
