 Aldehydes Aldehydes Ketones and Carboxylic Carboxylic Acids
Aldehydes Aldehydes Ketones and Carboxylic Carboxylic Acids
most important functional groups in organic chemistry. In aldehydes the carbonyl group is bonded They add fragrance and flavour to nature
 The Carbonyl Group Nomenclature of Aldehydes and Ketones
The Carbonyl Group Nomenclature of Aldehydes and Ketones
the parent compound benzaldehyde. (The carbon to which the aldehyde group is attached is carbon “1”). 10. Examples: Naming Aldehydes and Ketones.
 INFRARED SPECTROSCOPY (IR)
INFRARED SPECTROSCOPY (IR)
Uses of the Infrared Spectrum (p. 847-853). • Look over pages 853-866 after viewing this presentation for additional examples of various functional groups.
 New Aldehyde?Functional Methacrylic Water?Soluble Polymers
New Aldehyde?Functional Methacrylic Water?Soluble Polymers
there are no literature examples of water-soluble aldehyde- functional vinyl monomers. Aldehydes are extremely useful functional groups in.
 Infrared Spectroscopy
Infrared Spectroscopy
15 mai 2013 The exact wavenumber of the C=O stretch can give you clues as to whether the compound is a ketone aldehyde
 Short Summary of IUPAC Nomenclature of Organic Compounds
Short Summary of IUPAC Nomenclature of Organic Compounds
Nomenclature of Molecules Containing Substituents and Functional Groups hydroxy- amino-. Structure. Family of Compound. Carboxylic Acid. Aldehyde.
 Aldehydes Aldehydes Ketones and Carboxylic Acids Aldehydes
Aldehydes Aldehydes Ketones and Carboxylic Acids Aldehydes
most important functional groups in organic chemistry. In aldehydes the carbonyl group is bonded They add fragrance and flavour to nature
 Subject: Chemistry Synthesis Key features & characteristics
Subject: Chemistry Synthesis Key features & characteristics
2 Example of making an aldehyde. Oxidation of primary alcohol to aldehyde group. Isomerism. Aldehydes and ketones are functional group.
 1.1 Functional Groups of Biomolecules and their Reactions
1.1 Functional Groups of Biomolecules and their Reactions
1) which contributes to the reactivity of the compounds that have this functional group (aldehydes
 New Aldehyde?Functional Methacrylic Water?Soluble Polymers
New Aldehyde?Functional Methacrylic Water?Soluble Polymers
there are no literature examples of water-soluble aldehyde- functional vinyl monomers. Aldehydes are extremely useful functional groups in.
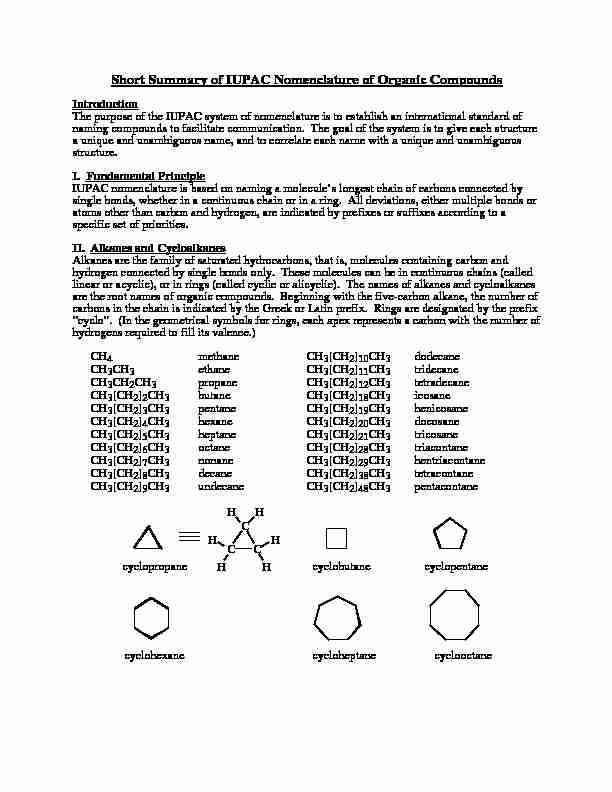 Short Summary of IUPAC Nomenclature of Organic Compounds
Short Summary of IUPAC Nomenclature of Organic Compounds IntroductionThe purpose of the IUPAC system of nomenclature is to establish an international standard ofnaming compounds to facilitate communication. The goal of the system is to give each structurea unique and unambiguous name, and to correlate each name with a unique and unambiguousstructure.
I. Fundamental Principle IUPAC nomenclature is based on naming a molecule's longest chain of carbons connected bysingle bonds, whether in a continuous chain or in a ring. All deviations, either multiple bonds oratoms other than carbon and hydrogen, are indicated by prefixes or suffixes according to aspecific set of priorities.
II. Alkanes and CycloalkanesAlkanes are the family of saturated hydrocarbons, that is, molecules containing carbon andhydrogen connected by single bonds only. These molecules can be in continuous chains (calledlinear or acyclic), or in rings (called cyclic or alicyclic). The names of alkanes and cycloalkanesare the root names of organic compounds. Beginning with the five-carbon alkane, the number ofcarbons in the chain is indicated by the Greek or Latin prefix. Rings are designated by the prefix"cyclo". (In the geometrical symbols for rings, each apex represents a carbon with the number ofhydrogens required to fill its valence.)
CH4methaneCH3[CH2]10CH3dodecaneCH
3CH3ethaneCH3[CH2]11CH3tridecaneCH
3CH2CH3propaneCH3[CH2]12CH3tetradecaneCH
3[CH2]2CH3butaneCH3[CH2]18CH3icosaneCH
3[CH2]3CH3 pentaneCH3[CH2]19CH3henicosaneCH
3[CH2]4CH3hexaneCH3[CH2]20CH3docosaneCH
CHH H HHHShort Summary of IUPAC Nomenclature, p. 2
III. Nomenclature of Molecules Containing Substituents and Functional GroupsA. Priorities of Substituents and Functional GroupsLISTED HERE FROM HIGHEST TO LOWEST PRIORITY, except that the substituents withinGroup C have equivalent priority.
Family of Compound
Alkene
AlkyneStructurePrefix
----Suffix -ene -yneSuffix -oic acid (-carboxylic acid) -al(carbaldehyde) -one -ol -aminePrefix carboxy- oxo- (formyl) oxo- hydroxy- amino-StructureFamily of CompoundCarboxylic Acid
Aldehyde
Ketone
Alcohol
AmineRCO
OH CHO R RCO R OH NR R CC CCGroup A - Functional Groups Indicated By Prefix Or Suffix Group B - Functional Groups Indicated By Suffix Only Group C - Substituents Indicated by Prefix Only Substituent Structure Prefix SuffixAlkyl (see list below)R - alkyl-------AlkoxyR - O - alkoxy-------HalogenF - fluoro---------Cl - chloro---------Br - bromo---------I - iodo---------Group C continued on next page
Short Summary of IUPAC Nomenclature, p. 3
Group C - Substituents, continued
Miscellaneous substituents and their prefixes NO 2CHCH 2CHCH 2CH2nitrovinylallylphenyl
Common alkyl groups - replace "ane" ending of alkane name with "yl". Alternate names forcomplex substituents are given in brackets.
methyl ethyl propyl (n-propyl)butyl (n-butyl)isopropyl [1-methylethyl]isobutyl [2-methylpropyl]sec-butyl[1-methylpropyl]tert-butyl or t-butyl[1,1-dimethylethyl]CH 3CH2CH3CH
2CH2CH3CH
2CH2CH2CH3CH
CH 3CH 3CHCH 3CH 3CH 2CH CH2CH3CH
3CCH 3CH 3CH3B. Naming Substituted Alkanes and Cycloalkanes - Group C Substituents Only
1. Organic compounds containing substituents from Group C are named following this sequenceof steps, as indicated on the examples below:•Step 1. Find the longest continuous carbon chain. Determine the root name for thisparent chain. In cyclic compounds, the ring is usually considered the parent chain, unless it isattached to a longer chain of carbons; indicate a ring with the prefix "cyclo" before the rootname. (When there are two longest chains of equal length, use the chain with the greater numberof substituents.)•Step 2. Number the chain in the direction such that the position number of the firstsubstituent is the smaller number. If the first substituents from either end have the same number,then number so that the second substituent has the smaller number, etc.•Step 3. Determine the name and position number of each substituent. (A substituent ona nitrogen is designated with an "N" instead of a number; see Section III.D.1. below.)•Step 4. Indicate the number of identical groups by the prefixes di, tri, tetra, etc.•Step 5. Place the position numbers and names of the substituent groups, in alphabeticalorder, before the root name. In alphabetizing, ignore prefixes like sec-, tert-, di, tri, etc., butinclude iso and cyclo. Always include a position number for each substituent, regardless ofredundancies.
Short Summary of IUPAC Nomenclature, p. 4
Examples
1-sec-butyl-3-nitrocyclohexane(numbering determined by the alphabetical order of substituents)3-fluoro-4-isopropyl-2-methylheptane3-bromo-2-chloro-5-ethyl-4,4-dimethyloctane766
4554
13 32
2187
6 5 4321
CHCH 2CH3H 3CNO
2CHCHCH
3CHCH2CH2CH3CH
3CH3CHCHC
BrCH 3CHCH2CH2CH3CH
CH 3CH 3ClCH2CH3CH
3F C. Naming Molecules Containing Functional Groups from Group B - Suffix Only1. Alkenes - Follow the same steps as for alkanes, except:
a. Number the chain of carbons that includes the C=C so that the C =C has the lowerposition number, since it has a higher priority than any substituents;
b. Change "ane" to "ene" and assign a position number to the first carbon of the C =C;c. Designate geometrical isomers with a cis,trans or E,Z prefix. 1
2345buta-1,3-diene5-methylcyclopenta-
1,3-dieneCHCH
2CHCHF
FCH 3C CH 3C FFquotesdbs_dbs2.pdfusesText_2[PDF] aldehyde functional group ir
[PDF] aldehyde functional group ir spectrum
[PDF] aldehyde functional group name
[PDF] aldehyde functional group properties
[PDF] aldehyde functional group suffix
[PDF] aldehyde hydrolysis
[PDF] aldehyde ir spectrum
[PDF] aldehyde ketone and carboxylic acid mcq pdf
[PDF] aldehyde ketone and carboxylic acid notes for neet pdf
[PDF] aldehyde ketone and carboxylic acid notes in hindi
[PDF] aldehyde ketone and carboxylic acid notes pdf download
[PDF] aldehyde ketone and carboxylic acid pdf target
[PDF] aldehyde ketone and carboxylic acid questions pdf
[PDF] aldehyde to ketone
