 Aldehydes Aldehydes Ketones and Carboxylic Carboxylic Acids
Aldehydes Aldehydes Ketones and Carboxylic Carboxylic Acids
most important functional groups in organic chemistry. In aldehydes the carbonyl group is bonded They add fragrance and flavour to nature
 The Carbonyl Group Nomenclature of Aldehydes and Ketones
The Carbonyl Group Nomenclature of Aldehydes and Ketones
the parent compound benzaldehyde. (The carbon to which the aldehyde group is attached is carbon “1”). 10. Examples: Naming Aldehydes and Ketones.
 INFRARED SPECTROSCOPY (IR)
INFRARED SPECTROSCOPY (IR)
Uses of the Infrared Spectrum (p. 847-853). • Look over pages 853-866 after viewing this presentation for additional examples of various functional groups.
 New Aldehyde?Functional Methacrylic Water?Soluble Polymers
New Aldehyde?Functional Methacrylic Water?Soluble Polymers
there are no literature examples of water-soluble aldehyde- functional vinyl monomers. Aldehydes are extremely useful functional groups in.
 Infrared Spectroscopy
Infrared Spectroscopy
15 mai 2013 The exact wavenumber of the C=O stretch can give you clues as to whether the compound is a ketone aldehyde
 Short Summary of IUPAC Nomenclature of Organic Compounds
Short Summary of IUPAC Nomenclature of Organic Compounds
Nomenclature of Molecules Containing Substituents and Functional Groups hydroxy- amino-. Structure. Family of Compound. Carboxylic Acid. Aldehyde.
 Aldehydes Aldehydes Ketones and Carboxylic Acids Aldehydes
Aldehydes Aldehydes Ketones and Carboxylic Acids Aldehydes
most important functional groups in organic chemistry. In aldehydes the carbonyl group is bonded They add fragrance and flavour to nature
 Subject: Chemistry Synthesis Key features & characteristics
Subject: Chemistry Synthesis Key features & characteristics
2 Example of making an aldehyde. Oxidation of primary alcohol to aldehyde group. Isomerism. Aldehydes and ketones are functional group.
 1.1 Functional Groups of Biomolecules and their Reactions
1.1 Functional Groups of Biomolecules and their Reactions
1) which contributes to the reactivity of the compounds that have this functional group (aldehydes
 New Aldehyde?Functional Methacrylic Water?Soluble Polymers
New Aldehyde?Functional Methacrylic Water?Soluble Polymers
there are no literature examples of water-soluble aldehyde- functional vinyl monomers. Aldehydes are extremely useful functional groups in.
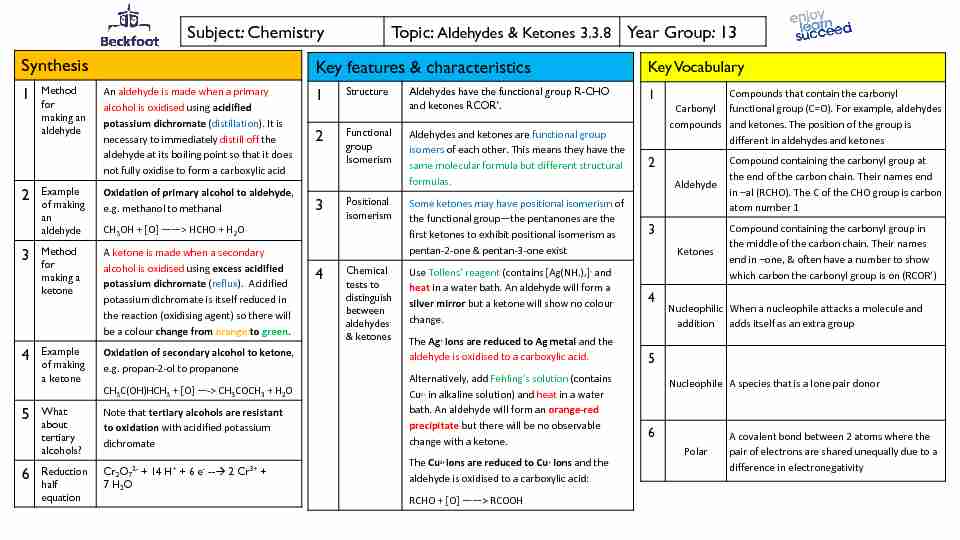 Subject: ChemistryTopic: Aldehydes & Ketones 3.3.8Year Group: 13
Subject: ChemistryTopic: Aldehydes & Ketones 3.3.8Year Group: 13 Synthesis
1Method
for making an aldehydeAn aldehyde is made when a primary
alcohol is oxidisedusing acidified potassium dichromate (distillation). It is necessary to immediately distill off the aldehyde at its boiling point so that it does not fully oxidiseto form a carboxylic acid2Example
of making an aldehydeOxidation of primary alcohol to aldehyde,
e.g.methanol to methanalCH3OH + [O] ͶͶ> HCHO + H2O
3Method
for making a ketoneA ketone is made when a secondary
alcohol is oxidisedusing excess acidified potassium dichromate (reflux). Acidified potassium dichromate is itself reduced in the reaction (oxidisingagent) so there will be a colourchange from orangeto green.4Example
of making a ketoneOxidation of secondary alcohol to ketone,
e.g.propan-2-ol to propanoneCH3C(OH)HCH3+ [O] Ͷ-> CH3COCH3+ H2O
5What about tertiary alcohols?Note that tertiary alcohols are resistant
to oxidation with acidified potassium dichromate6Reduction
half equationCr2O72-+ 14 H++ 6 e---2 Cr3++
7 H2OKey features & characteristics
1StructureAldehydes have the functional group R-CHO
2Functional
groupIsomerism
Aldehydes and ketones are functional group
isomers of each other. This means they have the same molecular formula but different structural formulas.3Positional
isomerismSome ketones may have positional isomerism of
the functional groupͶthe pentanones are the first ketones to exhibit positional isomerism as pentan-2-one & pentan-3-one exist4Chemical
tests to distinguish between aldehydes & ketones Use ŽůůĞŶƐ͛ƌĞĂŐĞŶƚ(contains [Ag(NH3)2]+and heat in a water bath. An aldehyde will form a silver mirrorbut a ketone will show no colour change.The Ag+ions are reduced to Ag metal and the
aldehyde is oxidisedto a carboxylic acid. Alternatively, add ĞŚůŝŶŐ͛ƐƐŽůƵƚŝŽŶ(containsCu2+in alkaline solution) and heat in a water
bath. An aldehyde will form an orange-red precipitatebut there will be no observable change with a ketone.The Cu2+ ions are reduced to Cu+ions and the
aldehyde is oxidisedto a carboxylic acid:RCHO + [O] ͶͶ> RCOOH
Key Vocabulary
1Carbonyl
compoundsCompounds that contain the carbonyl
functional group (C=O). For example, aldehydes and ketones. The position of the group is different in aldehydes and ketones 2Aldehyde
Compound containing the carbonyl group at
the end of the carbon chain. Their names end in ʹal (RCHO). The C of the CHO group is carbon atom number 1 3Ketones
Compound containing the carbonyl group in
the middle of the carbon chain. Their names end in ʹone, & often have a number to show4Nucleophilic
additionWhen a nucleophile attacks a molecule and
adds itself as an extra group 5NucleophileA species that is a lone pair donor
6 PolarA covalent bond between 2 atoms where the
pair of electrons are shared unequally due to a difference in electronegativity Subject: ChemistryTopic: Aldehydes & KetonesYear Group: 13Making carboxylic acids from an aldehyde
1Method to convert
aldehyde to carboxylic acid If you fully oxidise a primary alcohol using excess acidified potassiumdichromate and reflux or ŽdžŝĚŝƐĞĂŶĂůĚĞŚLJĚĞǁŝƚŚŽůůĞŶƐ͛ƌĞĂŐĞŶƚŽƌ
ĞŚůŝŶŐ͛ƐƐŽůƵƚŝŽŶ, you will produce the corresponding carboxylic acid
2Example equationCH3CHO + [O] ---CH3COOH
[O] represents oxidising agent3Example half-equationCH3CH2OH + H2O ͶͶͶ-> CH3COOH + 4H++ 4e-
Why do nucleophiles attack
aldehydes & ketones? 1 with equal probability from above and below..3..so that equal amounts of each enantiomer are
formed (racemate).4The racemate has no effect on plane polarised
lightas each enantiomer rotates the plane of plane polarisedlight in opposite directions but by equal amounts so that there is no overall effect (optically inactive).Nucleophilcaddition (reduction)
1Reagent & conditionsSodium tetrahydridoborate(III) (NaBH4) in aqueous methanol
2NucleophileThe nucleophile is the hydride ion (H-)
3ProductsThe aldehydeŝƐƌĞĚƵĐĞĚƚŽĨŽƌŵŝƚ͛ƐĐŽƌƌĞƐƉŽŶĚŝŶŐPRIMARY ALCOHOL
and the ketoneis reduced to form its corresponding SECONDARY ALCOHOL4Example
5Mechanism examples
with ethanal and propanoneReactions & Mechanisms
1Aldehydes & ketones both undergo
NUCLEOPHILIC ADDITION
mechanisms2This is because the C=O (carbonyl)
bond is polar due to oxygen having a greater electronegativity compared to carbon. This means the electron deficient carbon atomis susceptible to attack by a nucleophile.3This is an addition reaction because
the double C=O bond opens up to add the nucleophile into the molecule.4There are 2 reactions to remember: a)
REDUCTIONusing NaBH4to make
alcohols and b) reaction with KCNto make a hydroxynitrileNucleophilic addition (with KCN)
Reflux with aqueous
alcoholicKCN. The nucleophile is CN-. The product is aHYDROXYNITILE. HCN
quotesdbs_dbs2.pdfusesText_2[PDF] aldehyde functional group ir
[PDF] aldehyde functional group ir spectrum
[PDF] aldehyde functional group name
[PDF] aldehyde functional group properties
[PDF] aldehyde functional group suffix
[PDF] aldehyde hydrolysis
[PDF] aldehyde ir spectrum
[PDF] aldehyde ketone and carboxylic acid mcq pdf
[PDF] aldehyde ketone and carboxylic acid notes for neet pdf
[PDF] aldehyde ketone and carboxylic acid notes in hindi
[PDF] aldehyde ketone and carboxylic acid notes pdf download
[PDF] aldehyde ketone and carboxylic acid pdf target
[PDF] aldehyde ketone and carboxylic acid questions pdf
[PDF] aldehyde to ketone
