 Crystal Field Theory
Crystal Field Theory
The splitting of d orbital energies and its consequences are at the heart of crystal field theory. Page 5. 5. CFT-Octahedral Complexes. •For the Oh
 Crystal Field Theory (CFT)
Crystal Field Theory (CFT)
19-Dec-2018 As originally developed crystal field theory was used to describe the electronic structure of metal ions in crystals
 Untitled
Untitled
Crystal field Theory (CFT):. Developed by H. Bethe and van. Vleck. Originally crystal field theory was applied to transition metal ions in ionic crystals.
 MSCCH-17/18/19 Course Code-CHE-501 Unit- 6 Crystal Field
MSCCH-17/18/19 Course Code-CHE-501 Unit- 6 Crystal Field
CRYSTAL FIELD THEORY (CFT). In view of the weaknesses of Valence Bond Theory (VBT) an alternative bonding model was applied to transition metal complexes.
 Crystal Field Theory
Crystal Field Theory
❖ Electric field generated by the ligands influences the distribution of electrons in the metal ions i.e. d-orbital splitting. ❖ The bonding between the
 CRYSTAL FIELD THEORY (CFT)
CRYSTAL FIELD THEORY (CFT)
Crystal Field Theory was proposed by the physicist Hans Bethe in 1929 to describe the bonding in coordination complexes and to rationalize and predict some
 Coordination Compounds
Coordination Compounds
(vi) It does not distinguish between weak and strong ligands. The crystal field theory (CFT) is an electrostatic model which considers the metal-ligand bond to
 CRYSTAL FIELD THEORY (CFT) This theory advanced by Bethe
CRYSTAL FIELD THEORY (CFT) This theory advanced by Bethe
7) Different crystal fields will have different effects on the relative energies of the five d orbitals. Crystal field splitting of d-orbitals: The out come of
 Crystal Field Theory (CFT)
Crystal Field Theory (CFT)
Crystal Field Theory (CFT). The bonding of transition metal complexes can be explained by two approaches: crystal field theory and molecular orbital theory.
 Crystal Field Theory
Crystal Field Theory
Crystal field theory. (CFT) is a bonding model that explains many important properties of transition-metal complexes including their colors
 Crystal Field Theory
Crystal Field Theory
The splitting of d orbital energies and its consequences are at the heart of crystal field theory. Page 5. 5. CFT-Octahedral Complexes. •For the Oh
 Crystal Field Theory (CFT)
Crystal Field Theory (CFT)
Dec 19 2018 As originally developed
 CRYSTAL FIELD THEORY (CFT)
CRYSTAL FIELD THEORY (CFT)
Crystal Field Theory was proposed by the physicist Hans Bethe in 1929 to describe the bonding in coordination complexes and to rationalize and predict some
 Topic: Crystal Field Theory (CFT)
Topic: Crystal Field Theory (CFT)
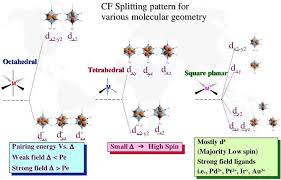
The crystal field splitting is measured in terms of energy difference between t2g and eg orbital and is denoted by a symbol ?o . It is generally measured
 Chemistry Notes for class 12 Chapter 9 Coordination Compounds .pdf
Chemistry Notes for class 12 Chapter 9 Coordination Compounds .pdf
By using spectroscopic data for a number of coordination compounds having the same metal ions but different ligand
 Coordination Compounds
Coordination Compounds
(vi) It does not distinguish between weak and strong ligands. The crystal field theory (CFT) is an electrostatic model which considers the metal-ligand bond to
 ATOICV1-7-1-Limitation-of-Crystal-Field-Theory.pdf
ATOICV1-7-1-Limitation-of-Crystal-Field-Theory.pdf
The main drawback of the crystal field theory is that it does not consider the covalent character in metal-ligand bonding at all. It treats the metal-ligand
 Crystal Field Theory
Crystal Field Theory
Crystal field theory. (CFT) is a bonding model that explains many important properties of transition-metal complexes including their colors
 Crystal Field Splitting in an Octahedral Field
Crystal Field Splitting in an Octahedral Field
as e. The crystal field splitting in the tetrahedral field is intrinsically smaller than in the octahedral field For most purposes the relationship may be
 B.Sc. III YEAR INORGANIC CHEMISTRY-III
B.Sc. III YEAR INORGANIC CHEMISTRY-III
2.4 AN ELEMENTARY IDEA OF CRYSTAL FIELD THEORY. In view of the above weaknesses an alternative bonding model was applied to transition metal complexes.
2018/2019 Coordination Chemistry Dr. Asia Hameed
1Crystal Field Theory (CFT)
As originally developed, crystal field theory was used to describe the electronic structure of metal ions in crystals, where they are surrounded by oxide ions or other anions that create an electrostatic field with symmetry dependent on the crystal structure. The energies of the d orbitals of the metal ions are split by the electrostatic field, and approximate values for these energies can be calculated. CFT was developed in 1930s. Shortly afterward, it was recognized that the same arrangement ofcharged or neutral electron pair donor species around a metal ion existed in crystals and
coordination complexes. In order to understand clearly the interactions that are responsible for crystal or ligand field effects in transition metal complexes, it is necessary to know the geometrical relationships of the d orbitals. There are five wave functions that can be written for orbitals having the typical four-lobed form.2018/2019 Coordination Chemistry Dr. Asia Hameed
2Crystal Field Effects in Octahedral Complexes
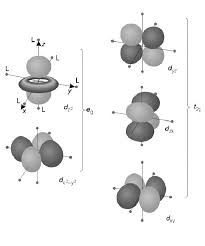
When the d orbitals of a metal ion are placed in an octahedral field of ligand electron pairs, directed at the surrounding ligands, are raised in energy. The dxy, dxz, and dyz orbitals, which are directed between the surrounding ions, are relatively unaffected by the field. The resulting energy difference is identified as οo( o for octahedral; some older references use the term 10Dq instead of οo). In case of free metal ion all the five d-orbitals are degenerate (these have the same energy). Now consider an octahedral complex, [ML6]n+ in which the central metal cation, Mn+ is placed at the center of the octahedral and is surrounded by six ligands which reside at the six corners of the octahedral. Now suppose both the ligands on each of the three axes are allowed to approach towards the metal cation, Mn+ from both the ends of the axes. In this process the electrons in d-orbitals of the metal cation are repelled by the negative point charge or by the negative end of the dipole of the ligands. (Remember CFT the ionic ligands as negative point charges and neutral ligands asdipoles). This repulsion will raise the energy of all the five d-orbitals. Since the lobes of dz2 and
dx2-y2 orbitals (eg orbitals) lie directly in the path of the approaching ligands, the electrons in these orbitals experience greater force of repulsion than those in dxy, dyz, and dzx orbitals2018/2019 Coordination Chemistry Dr. Asia Hameed
3 (t2g orbitals) whose lobes are directed in space between the path of the approaching ligands (theenergy of eg orbitals is increased while that of t2g is decreased (greater the repulsion, greater the
increase in energy). Thus we find that under the influence of approaching ligands, the five d-orbitals which were originally degenerate in free metallic cation are now split (or resolved) into two levels, t2g level which is triply degenerate and is of lower energy, and eg level which is doubly degenerate and is of higher energy. The resulting energy difference is identified as οo (o for octahedral) or 10Dq. This approach provides a simple means of identifying the d- orbital splitting found in coordination complexes and can be extended to include more quantitative calculations.2018/2019 Coordination Chemistry Dr. Asia Hameed
4 To gain some appreciation for the magnitude of οo and how it may be measured, let us consider the d1 complex, [Ti(H2O)6]3+. This ion exists in aqueous solution of Ti3+ and gives rise to a purple color. The single d electron in the complex will occupy the lowest energy orbitalavailable to it (one of the three degenerate t2g orbitals). The purple color is the result of
absorption of light and promotion of the t2g electron to the eg level. The transition can be represented as t12geg \t02ge1g The absorption spectra of [Ti(H2O)6]3+ reveals that this transition occurs with a maximum at20300 cm-1 of energy for οo.
The d1 case is the simplest possible because the observed spectral transition reflects the actual energy difference between the eg and t2g levels.Crystal Field Stabilization Energy (CFSE)
In the d1 case discussed above , the electron occupies a t2g orbital, which has an energy of- 0.4 οo relative to the barycenter of the d orbitals. The complex can thus be said to be stabilized
to the extent of 0.4 οo compared to the hypothetical spherical-field case. This quantity is termed
the crystal field stabilization energy (CFSE).2018/2019 Coordination Chemistry Dr. Asia Hameed
5For d2 and d3
different degenerate t2g orbitals and to remain unpaired. The resulting configuration, t22g and t32g,
will have CFSE of - 0.8 quotesdbs_dbs14.pdfusesText_20[PDF] cse 2221 osu reddit
[PDF] cse 2221 project 10
[PDF] cse 2221 sequence
[PDF] cse 2221 syllabus
[PDF] cse 2231
[PDF] cse 2321
[PDF] cse2221 homepage
[PDF] cspire
[PDF] cspire customer service contact number
[PDF] cspire customer service email
[PDF] cspire customer service jobs
[PDF] cspire customer service pay bill
[PDF] cspire customer service text
[PDF] css cheat sheet codecademy
