 osmosis-demo-lab.pdf
osmosis-demo-lab.pdf
4) Observe plasmolysis in Elodea. Background. Osmosis is the process whereby water moves across a cell membrane by diffusion. Diffusion takes place when the
 DIFFUSION AND OSMOSIS
DIFFUSION AND OSMOSIS
• ESSENTIAL FOR CELLS. • WATER MOVES ACROSS THE CELL MEMBRANE BY OSMOSIS. IT DEPENDS ON THE CONCENTRATION OF WATER INSIDE AND OUTSIDE. THE CELL. Page 12. Follow
 Reverse Osmosis Concentrate Treatment Research Results and
Reverse Osmosis Concentrate Treatment Research Results and
Nov 19 2020 Although the ozone and open-water treatment cells did not remove all contaminants
 DIFFUSION AND OSMOSIS
DIFFUSION AND OSMOSIS
Most cells achieve this control by delicate cell membranes that can often regulate different substances by slowing down the movement of some molecules while
 An Overview of Osmosis Study in Living Cells and its Implication in
An Overview of Osmosis Study in Living Cells and its Implication in
Apr 3 2022 It was found in the case of water movements across the cell membranes [4]
 Cellular Processes: Energy and Communication
Cellular Processes: Energy and Communication
In walled cells osmosis is affected not only by the solute concentration but also by the resistance to water movement in the cell by the cell wall. This
 Osmosis and Osmoregulation
Osmosis and Osmoregulation
• Water moves in and out of cells. →Cells swell or shrink. • This has profound effects on the brain. – Neurologic function is altered. – Rapid shrinking can
 4 Homeostasis and Cell Transport.pdf
4 Homeostasis and Cell Transport.pdf
through osmosis and diffusion. The cell membrane also separates the cell's internal environment from the external world. Page 20
 Effect of Immersion Time in Osmosis and Ultrasound on Papaya Cell
Effect of Immersion Time in Osmosis and Ultrasound on Papaya Cell
Feb 9 2009 Ultrasound-assisted osmotic dehydration induced a gradual distortion in the shape of the cells
 Measuring osmosis and hemolysis of red blood cells
Measuring osmosis and hemolysis of red blood cells
No change in cell volume occurs in isotonic NaCl and
 Gummy Bear Chemistry and Osmosis
Gummy Bear Chemistry and Osmosis
The Cell Membrane. • Cells are surrounded by a lipid bilayer which is a semi-permeable membrane. • Semi-permeable means that only.
 What is the difference between osmosis and diffusion?
What is the difference between osmosis and diffusion?
Examples of Diffusion: Examples of diffusion include perfume filling a whole room and the movement of small molecules across a cell membrane. One of the
 osmosis-demo-lab.pdf
osmosis-demo-lab.pdf
The effect of water loss on plant cells is shown in the diagram below. Figure 1. A. Plant cell placed in pure water. This cell will become inflated because the
 Measuring osmosis and hemolysis of red blood cells
Measuring osmosis and hemolysis of red blood cells
No change in cell volume occurs in isotonic NaCl and
 12 4 ELECTRO-OSMOSIS IN CHARA AND NITELLA CELLS P. H.
12 4 ELECTRO-OSMOSIS IN CHARA AND NITELLA CELLS P. H.
are used to reappraise the possibility that electro-osmosis in cells may be explained in terms of electro-osmotic coupling in cell walls alone. INTRODUCTION.
 DIFFUSION AND OSMOSIS
DIFFUSION AND OSMOSIS
Most cells achieve this control by delicate cell membranes that can often regulate different substances by slowing down the movement of some molecules while
 Bend a Carrot
Bend a Carrot
Learning Objectives: Students understand the process of osmosis This experiment investigates the movement of water into and out of cells. The.
 Cellular Processes: Energy and Communication
Cellular Processes: Energy and Communication
In walled cells osmosis is affected not only by the solute concentration but also by the resistance to water movement in the cell by the cell wall. This
 Zeta-Potentials of Intact Cell Monolayers Determined by Electro
Zeta-Potentials of Intact Cell Monolayers Determined by Electro
Mar 14 2017 Key words: electrophoresis; electro-osmosis; Zeta potential; monolayers; viability. Introduction. The ??-potential of a cell surface as ...
 Another Lesson from Plants: The Forward Osmosis-Based Actuator
Another Lesson from Plants: The Forward Osmosis-Based Actuator
water-driven processes in plant cells and tissues and thus can be used as guide for designing osmosis-based actuators in a biomimetic approach.
 Lab 3 Osmosis: How Does the Concentration of Salt in Water
Lab 3 Osmosis: How Does the Concentration of Salt in Water
Osmosis refers specifically to the diffusion of water molecules In cells water cannot simply diffuse FIGURE L3 2 across the membrane However special openings in the (a) Red blood cells in saltwater solution membrane allow for easy flow of water molecules so cells can and (b) normal red blood cells take in or get rid of water when needed
 DIFFUSION AND OSMOSIS
DIFFUSION AND OSMOSIS
1 Define osmosis 2 Understand the concept of water potential gradient 3 Understand the concept of hypotonic isotonic and hypertonic solutions 4 Understand how animals and plant cells respond to immersion in solutions which are of different concentration to their cytoplasm
 Why is osmosis important to the survival of a cell?
Why is osmosis important to the survival of a cell?
Lab 4: Osmosis and Diffusion The plasma membrane enclosing every cell is the boundary that separates the cell from its external environment It is not an impermeable barrier but like all biological membranes is selectively permeable controlling which molecules move into and out of the cell
 DIFFUSION AND OSMOSIS - Yavapai College
DIFFUSION AND OSMOSIS - Yavapai College
Osmosis is the movement of water through a selectively permeable membrane Flow movement is dependent upon the concentration of dissolved substances (solutes) on either side of the membrane Consequently water passes from an area of high concentration of water molecules to an area of low concentration of water molecules
 Lab 3: Osmosis in Model & Living Cells Objectives: To - WRUV
Lab 3: Osmosis in Model & Living Cells Objectives: To - WRUV
Osmosis: the diffusion of water molecules across a semi-permeable membrane from an area of greater concentration of water to an area of lesser concentration of water Waterpotential:a measure of the chemical potential of water molecules A combination of osmotic and pressure potentials (see p 3)
 Searches related to osmosis in cells filetype:pdf
Searches related to osmosis in cells filetype:pdf
The processes of diffusion osmosis and filtration are responsible for the movement of materials into and out of body cells as well as the exchange of molecules between body fluid compartments These processes involve some basic principles of physics which will be demonstrated in this laboratory
Why is osmosis important to the survival of a cell?
- This obviously is essential to the survival of a cell. The most important function of osmosis is stabilising the internal environment of an organism by keeping the water and intercellular fluids levels balanced. In all living organisms, nutrients and minerals make their way to the cells because of osmosis.
What factors determines the osmosis of a cell?
- Factors that Affect Osmosis. Concentration gradient: The greater the concentration difference, the faster the rate of osmosis. Temperature: The higher the temperature, the faster the rate of osmosis Example of osmosis in a living cell (human being) In the human body system, the kidney cleans the blood by filtering out waste substances.
How do osmosis help the cell maintain homeostasis?
- Osmosis is the diffusion of water. Osmosis helps cells maintain homeostasis because a cell needs to maintain a specific water balance so that chemical reactions can take place. Osmosis can also be used to balance out the concentration of other molecules (such as sugar or salt) present on either side of a cell membrane.
62210 Diffusion & Osmosis Lab 8-1 DIFFUSION AND OSMOSIS Introduction: The living cells that make up organisms remain alive as long as they are able to maintain proper physical and chemical states with their surrounding environment. It is often difficult to realize the ever-changing conditions that take place at the cellular level and the cell's ability to maintain a steady state. You may have, at one time or another, and possibly without knowing, experienced a change in cellular water balance if you have been exposed to long periods under the Arizona sun with minimum water. Cellular consistency, and thus organism constancy, is maintained by the regulation of materials into and out of cells. Most cells achieve this control by delicate cell membranes that can often regulate different substances by slowing down the movement of some molecules while allowing others to pass. Consequently, most cell membranes are said to be selectively permeable. In this exercise you will consider the basic processes that take place across membranes and the resulting consequences of various environmental conditions. Consider that the simple processes of diffusion and osmosis are basic for the relationships of more complex physiological functions such as respiration, digestion and water balance. Exercise #1 - Diffusion The movement of gases and dissolved substances from a region of high concentration to a region of low concentration is termed diffusion. Kinetic energy of molecules keeps the molecules in motion while the rate of diffusion depends on several factors. These factors include: molecule concentration, molecule size and molecule weight. Materials Needed: One agar petri plate One metric ruler Two forceps Potassium permanganate crystals Methylene blue crystals Wax pencil Procedure: With separate forceps, select out one very tiny crystal of potassium permanganate and one very tiny crystal of methylene blue. CAREFUL, THESE STAIN. Place one crystal of each chemical 3 cm apart on the agar petri plate. Observe and measure, in millimeters, the diameter of diffusion of the crystal molecules after 1-2 hours. The molecular weight of methylene blue is 374 and potassium permanganate is 158.
62210 Diffusion & Osmosis Lab 8-2 Name___________________________ Diffusion Report Sheet 1. Did the molecules move from an area of greater concentration to an area of lower concentration? Why? 2. Which chemical showed the greatest distance of diffusion? 3. Is there a correlation between molecular weight and the distance of diffusion? Explain. 4. Define diffusion:
62210 Diffusion & Osmosis Lab 8-3 Exercise #2 - Osmosis Osmosis is the movement of water through a selectively permeable membrane. Flow movement is dependent upon the concentration of dissolved substances (solutes) on either side of the membrane. Consequently, water passes from an area of high concentration of water molecules to an area of low concentration of water molecules. Conversely, water passes from an area of low solute (sucrose, salt...) concentration to an area of high solute (sucrose, salt...) concentration. Think of it in terms of the water trying to be the great equalizer, it moves in the direction necessary to make both sides of the membrane as equal as possible. If there is more solute on one side than the other, then the water will move from the area where it is more abundant (the area with lower solute concentration) to dilute out the area with the higher solute concentration (the area with lower water concentration). Materials Needed: Five dialysis (cellophane) tubes, 20 cm long Five plastic cups Wax pencil Bottle of distilled water Solutions: 10% sucrose, 20% sucrose, 30% sucrose and 60% sucrose Balance Procedure: 1. Fold one end of each dialysis tube over and tie a knot or use a clip to seal it. 2. Fill the tubes slightly more than 1/2 with the solutions indicated below. Tie the end with a knot or use a clip. Tube #1: distilled water Tube #2: 10% sucrose solution Tube #3: 20% sucrose solution Tube #4: 30% sucrose solution Tube #5: distilled water 3. Weigh each tube, in grams, and record the weight in Table 1. 4. Place tubes 1, 2, 3 and 4 in separate cups (label them) of distilled water. Place tube #5 in a cup of 60% sucrose solution. 5. Weigh each tube, in grams, at 15 minute intervals for one hour. Record all weights in Table 1. 6. Plot the results in Figure 1 and complete the questions.
62210 Diffusion & Osmosis Lab 8-4 Name___________________________ Osmosis Report Sheets Table 1. Osmosis Data Starting Weight Weight at 15 min. Weight at 30 min. Weight at 45 min. Weight at 60 min. Tube 1 Wt Difference: Wt Difference: Wt Difference: Wt Difference: Tube 2 Wt Difference: Wt Difference: Wt Difference: Wt Difference: Tube 3 Wt Difference: Wt Difference: Wt Difference: Wt Difference: Tube 4 Wt Difference: Wt Difference: Wt Difference: Wt Difference: Tube 5 Wt Difference: Wt Difference: Wt Difference: Wt Difference: Determine the total weight gain or loss, in grams, for each tube at the end of each 15 minute interval. To do this, subtract the starting weight from the total weight at the end of each 15 minute interval.
62210 Diffusion & Osmosis Lab 8-5 Figure 1. Osmosis Results Plot the total weight gain or loss, in grams, of each tube over time. Include a clearly labeled legend for the tubes. Use different colors for each tube or use different points ( ~ • ∞ ¢ ) for each tube. 1. Suppose you placed a tube containing 80% sucrose in a beaker of 40% sucrose. Would the tube gain or lose weight and why? 2. What would happen if you placed a tube with 40% sucrose in a beaker of 80% sucrose. Would the tube gain or lose weight and why? 3. Define osmosis: 0.0g Weight Gain (grams) Weight Loss (grams) 15 30 45 60 Time (minutes)
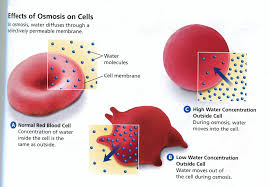
62210 Diffusion & Osmosis Lab 8-6 Exercise #3 - Hypotonic, Isotonic & Hypertonic Solutions The cell membrane is selectively permeable; some molecules pass freely through the cell membrane while others do not pass easily. We will now study the effects of diffusion of water from a region of high concentration to a region of low concentration across a cell membrane. This process is called osmosis. The cells of your body (red blood cells, skin, nerves...) have a salt concentration of 0.85% NaCl. This means your cells are 99.15% water (100% - 0.85% NaCl = 99.15% H2O). If you put a red blood cell in distilled water (100% H2O), there will be a net movement of water from a region of higher concentration (100%) to a region of lower concentration (the cell with 99.15% H2O). The distilled water outside the red blood cell, since it is 100% water and no salt, is hypotonic (it contains less salt than the red blood cell) to the red blood cell. The red blood cell will gain water, swell ad then burst. The bursting of the red blood cell is called hemolysis. Red blood cell in distilled water If a red blood cell is placed in a solution that contains 0.85% NaCl the water moves equally out and into the cell, the solution in the cell and the solution around the cell are the same or in equilibrium. There is no net gain or loss of water from the cell. The 0.85% NaCl solution outside the red blood cell is isotonic to the red blood cell. Red blood cell 0.85% NaCl If a red blood cell is placed in 2.0% NaCl (98% H2O) the red blood cell has 0.85% NaCl and 99.15% H2O. The salt solution now has 2.0% NaCl and 98% H2O (100% - 2.0% NaCl = 98% water). Water movement is from a higher concentration to a lower concentration. In this case water will move out of the red blood cell into the beaker. The red blood cell will lose water and will shrink. This shrinking is termed crenation or plasmolysis. The 2.0% NaCl solution outside the red blood cell is hypertonic (it contains more salt than the red blood cell) to the red blood cell. Red blood cell 2.0% NaCl
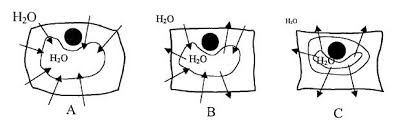
62210 Diffusion & Osmosis Lab 8-7 Name___________________________ Osmotic Relationships Report Sheet 1. The following diagrams represent beakers of water with varying concentrations of NaCl. Each beaker contains a cell with a NaCl content of 0.85%. Indicate, by using arrows, the direction of the net movement of water molecules. Also indicate the changes in cell shape and answer the questions below. 0.85% NaCl 0.00% NaCl 30% NaCl 2. What is the effect of 0.85% NaCl on the shape of the cell and why? 3. What is the effect of distilled water on the shape of the cell and why? 4. What is the effect of 30% NaCl on the shape of the cell and why? 5. Why would you become dehydrated by drinking a large amount of sea water? 5. Use the following three terms to fill in the blanks concerning the cell and beaker relationships on the previous page: hypotonic, isotonic and hypertonic. Distilled water is ______________________ to the cell. The cell is _________________________ to the 30% NaCl solution. The 0.85% NaCl solution is _____________________ to the cell. The cell is __________________________ to distilled water. The 30% NaCl solution is _______________________ to the cell.
62210 Diffusion & Osmosis Lab 8-8 Exercise #4 - Elodea & a Hypertonic Solution Materials Needed: Slide & coverslip One elodea leaf Bottle of distilled water A dropper bottle of 20% NaCl solution Procedure: 1. Make a wet mount of the elodea leaf in distilled water. 2. Observe the leaf under high power (400X) and draw one or two cells below. 3. Add a drop of 20% NaCl to the edge of the coverslip. 4. Replace the coverslip, observe the elodea leaf again at high power (400X) and draw one or two cells below. Distilled Water 20% NaCl
quotesdbs_dbs14.pdfusesText_20[PDF] osmosis worksheet answer key pdf
[PDF] osu cse components api
[PDF] osu cse components binary tree
[PDF] osu cse components stack
[PDF] osu cse documentation
[PDF] oswego ny newspapers online
[PDF] osxpmem
[PDF] other names for seven deadly sins
[PDF] otis 12 gauge shotgun cleaning kit
[PDF] otpf 3rd edition pdf
[PDF] ott business model pdf
[PDF] ottawa application login
[PDF] ottawa catholic school board calendar 2019 2020
[PDF] ottawa catholic school board strike
