 osmosis-demo-lab.pdf
osmosis-demo-lab.pdf
4) Observe plasmolysis in Elodea. Background. Osmosis is the process whereby water moves across a cell membrane by diffusion. Diffusion takes place when the
 DIFFUSION AND OSMOSIS
DIFFUSION AND OSMOSIS
• ESSENTIAL FOR CELLS. • WATER MOVES ACROSS THE CELL MEMBRANE BY OSMOSIS. IT DEPENDS ON THE CONCENTRATION OF WATER INSIDE AND OUTSIDE. THE CELL. Page 12. Follow
 Reverse Osmosis Concentrate Treatment Research Results and
Reverse Osmosis Concentrate Treatment Research Results and
Nov 19 2020 Although the ozone and open-water treatment cells did not remove all contaminants
 DIFFUSION AND OSMOSIS
DIFFUSION AND OSMOSIS
Most cells achieve this control by delicate cell membranes that can often regulate different substances by slowing down the movement of some molecules while
 An Overview of Osmosis Study in Living Cells and its Implication in
An Overview of Osmosis Study in Living Cells and its Implication in
Apr 3 2022 It was found in the case of water movements across the cell membranes [4]
 Cellular Processes: Energy and Communication
Cellular Processes: Energy and Communication
In walled cells osmosis is affected not only by the solute concentration but also by the resistance to water movement in the cell by the cell wall. This
 Osmosis and Osmoregulation
Osmosis and Osmoregulation
• Water moves in and out of cells. →Cells swell or shrink. • This has profound effects on the brain. – Neurologic function is altered. – Rapid shrinking can
 4 Homeostasis and Cell Transport.pdf
4 Homeostasis and Cell Transport.pdf
through osmosis and diffusion. The cell membrane also separates the cell's internal environment from the external world. Page 20
 Effect of Immersion Time in Osmosis and Ultrasound on Papaya Cell
Effect of Immersion Time in Osmosis and Ultrasound on Papaya Cell
Feb 9 2009 Ultrasound-assisted osmotic dehydration induced a gradual distortion in the shape of the cells
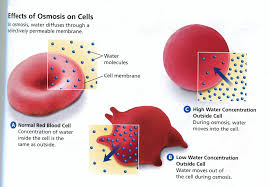
 Measuring osmosis and hemolysis of red blood cells
Measuring osmosis and hemolysis of red blood cells
No change in cell volume occurs in isotonic NaCl and
 Gummy Bear Chemistry and Osmosis
Gummy Bear Chemistry and Osmosis
The Cell Membrane. • Cells are surrounded by a lipid bilayer which is a semi-permeable membrane. • Semi-permeable means that only.
 What is the difference between osmosis and diffusion?
What is the difference between osmosis and diffusion?
Examples of Diffusion: Examples of diffusion include perfume filling a whole room and the movement of small molecules across a cell membrane. One of the
 osmosis-demo-lab.pdf
osmosis-demo-lab.pdf
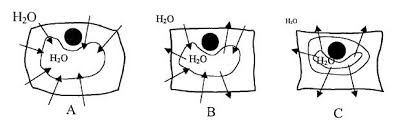
The effect of water loss on plant cells is shown in the diagram below. Figure 1. A. Plant cell placed in pure water. This cell will become inflated because the
 Measuring osmosis and hemolysis of red blood cells
Measuring osmosis and hemolysis of red blood cells
No change in cell volume occurs in isotonic NaCl and
 12 4 ELECTRO-OSMOSIS IN CHARA AND NITELLA CELLS P. H.
12 4 ELECTRO-OSMOSIS IN CHARA AND NITELLA CELLS P. H.
are used to reappraise the possibility that electro-osmosis in cells may be explained in terms of electro-osmotic coupling in cell walls alone. INTRODUCTION.
 DIFFUSION AND OSMOSIS
DIFFUSION AND OSMOSIS
Most cells achieve this control by delicate cell membranes that can often regulate different substances by slowing down the movement of some molecules while
 Bend a Carrot
Bend a Carrot
Learning Objectives: Students understand the process of osmosis This experiment investigates the movement of water into and out of cells. The.
 Cellular Processes: Energy and Communication
Cellular Processes: Energy and Communication
In walled cells osmosis is affected not only by the solute concentration but also by the resistance to water movement in the cell by the cell wall. This
 Zeta-Potentials of Intact Cell Monolayers Determined by Electro
Zeta-Potentials of Intact Cell Monolayers Determined by Electro
Mar 14 2017 Key words: electrophoresis; electro-osmosis; Zeta potential; monolayers; viability. Introduction. The ??-potential of a cell surface as ...
 Another Lesson from Plants: The Forward Osmosis-Based Actuator
Another Lesson from Plants: The Forward Osmosis-Based Actuator
water-driven processes in plant cells and tissues and thus can be used as guide for designing osmosis-based actuators in a biomimetic approach.
 Lab 3 Osmosis: How Does the Concentration of Salt in Water
Lab 3 Osmosis: How Does the Concentration of Salt in Water
Osmosis refers specifically to the diffusion of water molecules In cells water cannot simply diffuse FIGURE L3 2 across the membrane However special openings in the (a) Red blood cells in saltwater solution membrane allow for easy flow of water molecules so cells can and (b) normal red blood cells take in or get rid of water when needed
 DIFFUSION AND OSMOSIS
DIFFUSION AND OSMOSIS
1 Define osmosis 2 Understand the concept of water potential gradient 3 Understand the concept of hypotonic isotonic and hypertonic solutions 4 Understand how animals and plant cells respond to immersion in solutions which are of different concentration to their cytoplasm
 Why is osmosis important to the survival of a cell?
Why is osmosis important to the survival of a cell?
Lab 4: Osmosis and Diffusion The plasma membrane enclosing every cell is the boundary that separates the cell from its external environment It is not an impermeable barrier but like all biological membranes is selectively permeable controlling which molecules move into and out of the cell
 DIFFUSION AND OSMOSIS - Yavapai College
DIFFUSION AND OSMOSIS - Yavapai College
Osmosis is the movement of water through a selectively permeable membrane Flow movement is dependent upon the concentration of dissolved substances (solutes) on either side of the membrane Consequently water passes from an area of high concentration of water molecules to an area of low concentration of water molecules
 Lab 3: Osmosis in Model & Living Cells Objectives: To - WRUV
Lab 3: Osmosis in Model & Living Cells Objectives: To - WRUV
Osmosis: the diffusion of water molecules across a semi-permeable membrane from an area of greater concentration of water to an area of lesser concentration of water Waterpotential:a measure of the chemical potential of water molecules A combination of osmotic and pressure potentials (see p 3)
 Searches related to osmosis in cells filetype:pdf
Searches related to osmosis in cells filetype:pdf
The processes of diffusion osmosis and filtration are responsible for the movement of materials into and out of body cells as well as the exchange of molecules between body fluid compartments These processes involve some basic principles of physics which will be demonstrated in this laboratory
Why is osmosis important to the survival of a cell?
- This obviously is essential to the survival of a cell. The most important function of osmosis is stabilising the internal environment of an organism by keeping the water and intercellular fluids levels balanced. In all living organisms, nutrients and minerals make their way to the cells because of osmosis.
What factors determines the osmosis of a cell?
- Factors that Affect Osmosis. Concentration gradient: The greater the concentration difference, the faster the rate of osmosis. Temperature: The higher the temperature, the faster the rate of osmosis Example of osmosis in a living cell (human being) In the human body system, the kidney cleans the blood by filtering out waste substances.
How do osmosis help the cell maintain homeostasis?
- Osmosis is the diffusion of water. Osmosis helps cells maintain homeostasis because a cell needs to maintain a specific water balance so that chemical reactions can take place. Osmosis can also be used to balance out the concentration of other molecules (such as sugar or salt) present on either side of a cell membrane.
Lab 3: Osmosis in Model & Living Cells
Objectives: To simulate the osmotic behavior of a model cell. We will begin our study of the workings of plant cells by looking at water movement across semi-permeable membranes - osmosis - in model cells. In the second part of the lab, we will look at real plant cells. The movement of substances into and out of cells is accomplished largely by diffusion. In addition, much of the movement of substances within organisms, and specifically within cells is accomplished by diffusion. In fact, few, if any of the physiological processes occurring in cells are not directly or indirectly involved with the phenomenon of diffusion. Substances tend to diffuse from areas of higher concentration to areas of lower concentration of their kind. Another way to say that is they move from areas of higher chemical potential to areas of lower chemical potential. Chemical potential is a measure of the free energy available to do the work of moving a mole (6.02 x 10 23of molecules from one location to another and, in some cases, through a barrier such as a plasma membrane. In a solution, the greater the concentration of molecules of a dissolved substance (solute), the higher the chemical potential (free energy per mole) of that substance. Therefore, we say that molecules tend to move from areas of higher concentration (higher chemical potential) to areas of lower concentration (lower chemical potential). As you might remember, cells are bounded by a plasma membrane. All internal organelles of a plant cell (such as the nucleus, mitochondria, chloroplasts) are bounded by similar membranes. Water and dissolved solutes, such as sugars and ions, must traverse these membranes, but do so at vastly differing rates. Water can pass through the membrane easily, whereas other solutes cross the membrane only slowly, if at all; the membranes are semi-permeable. All substances that enter the cell are in solution in water and diffuse through the water. Diffusion of water molecules across a semi-permeable membrane is known as osmosis. Osmosis is, therefore, a special case of diffusion. Because of the differentially permeable properties of
KEY TERMS
Diffusion: the movement of molecules
from a region of higher concentration to a region of lower concentration.Chemical potential: a measure of the
free energy available to do the work of moving a mole of molecules (i.e., a certain number of atoms) from one location to another, sometimes through a membrane.Solute: the substance that is dissolved
(e.g., sugar).Solvent: the liquid in which substances
dissolve (e.g., water).Semi-permeable membrane: a
membrane through which different substances diffuse at different rates.Osmosis: the diffusion of water
molecules across a semi-permeable membrane from an area of greater concentration of water to an area of lesser concentration of water.Water potential: a measure of the
chemical potential of water molecules.A combination of osmotic and pressure
potentials (see p. 3). 2 the cell membranes there is a tendency for water to move across these membranes toward the solution with the higher solute concentration; or in terms of the water molecules, for the water molecules to move from a region of higher water potential to that of lower water potential. Water potential is a measure of the chemical potential, or free energy, of water molecules. Water potential is affected by the amount of solutes dissolved in the water. The more solute dissolved in the water, the lower the water potential. Pure water has the highest possible water potential.Water potential (P
w ) results from the combined actions of osmotic potential (or solute potential, Ψ s ), which is dependent on solute concentration in a solution, and pressure potential p ), which results from the exertion of positive pressure or negative pressure (tension) on a solution. We can express this relationship as: P w s p water osmotic pressure potential potential potential (Your book uses the Greek symbol Ψ ["Psi," pronounced "sigh"] instead of P when talking about water potential). Ø Movement of water always goes "downhill" from areas of high water potential to areas of lower water potential.Differences in water potential are a
measure of the tendency of water to leave one area in favor of another. The addition of solute to water lowers the osmotic potential of a solution (makes Ψ s more negative) and, therefore lowers the water potential of that solution. Cell membranes are permeable to water but often not to solutes, because solute molecules (e.g., sucrose or table sugar) are too big to pass through the pores of the permeable membrane. If there are more free water molecules on one side of the membrane than the other, there will be a net movement from high to low concentration (Figure 1). Thus, in the absence of other factors (such as pressure, Ψ p = 0), osmosis results in the net movement of water from an area of high water potential (low solute concentration) to an area of lower water potential (high solute concentration). Water potential on two sides of a differentially permeable membrane will eventually become equal if there are no limits to expansion.KEY TERMS
Osmotic potential: the pressure required to prevent osmosis. Pressure potential: the result of pressure (positive or negative) on a solution.Hypotonic: when the external solution has a lower
solute concentration than the cell.Isotonic: when the external solution has a solute
concentration equal to the cell. Hypertonic: when the external solution has a higher solute concentration than the cell, the solution is hypertonic relative to the cell. 3 When the external solution has a lower solute concentration than the cell, that solution is said to be hypotonic relative to the cell ("hypo" = "less than"). If the solute concentrations are equal, then the solution and the cell are isotonic ("iso" = "same"). Finally when the external solution has a higher solute concentration, then it is hypertonic ("hyper" = "more") relative to the cell (Figure 2). Figure 1. Osmosis - the movement of water molecules from a region of high water potential to a region of low water potentialFigure 2.
THOUGHT QUESTION
If a cell is placed in a hypotonic solution, which way will water move and why? 4 If a barrier (such as a cell wall) prevents expansion, then pressure builds up inside the compartment (cell) into which the water is moving. An increase in positive pressure raises the pressure potential (Ψ p ) and therefore the water potential inside the compartment; this decreases the water potential gradient (difference) between the solution outside the compartment and the solution inside the compartment. Water continues to flow into the cell until the difference is offset by increased pressure and a dynamic equilibrium is reached. At this point, the net flow of water molecules from outside to inside the cell stops. Water molecules continue to travel randomly back and forth but the number is equal in both directions.Experiment: Osmosis in a model cell
In this experiment, we will investigate the relationship between osmotic potential and solute concentration and between osmotic potential and the rate of movement of water through a semipermeable membrane by the process of osmosis. You will place dialysis tubes containing solutions of different sucrose (table sugar) concentrations into beakers containing distilled water (Figure 3). The rate and direction of water movement can be determined by weighing the tubes before and after placing them in distilled water; the dialysis tubes are permeable to water, but not to sucrose (as in Figure 1), and can gain or lose water. At atmospheric pressure, the water potential of pure water in the beakers can be assumed to be zero (P w = 0); no solute is present, thus osmotic potential is zero (P s = 0), and pressure potential is also zero (Ψ p ). The water potential of the sucrose solutions in the dialysis tubes will be negative (recall that the addition of solute to pure water decreases osmotic potential), thus, the water potentials of the solutions inside the tubes will be lower (P w is negative) than the water potential of the water outside the tubes, where P w is zero.THOUGHT QUESTION
Osmosis can be either beneficial or detrimental to a plant cell, depending on the circumstances. Under what circumstances is osmosis detrimental to the plant cell? Use Figure 2 to help answer this question. 5 Predict which way water will move under scenarios A and B:A. B.
Figure 3. We will be placing tubes made out of semipermeable membrane into two different solutions. See table (p. 8) for the sucrose concentrations that we will use.Experimental Procedure
SEE TABLE on p. 8 for details. Work in a group of five people.1) Soak six pieces of 15 cm long dialysis tubing in distilled water for a few minutes.
2) While the tubing soaks, label 6 clamps with masking tape: 5, 10, 20, 40, DI, DI.
3) Fold over one end of the tube 2 cm and clamp 1 cm from that end of the tube. Do not
let dialysis tubing dry out.4) Open the unclamped end of the tube and add 5 mL of the solution of your
experimental treatment. Then fold over the end, forcing out air, and clamp it. Make sure that there is space between the water and the clamp (but no air in the tube).5) Thoroughly rinse the tube with distilled water.
6) Weigh the tube on a scale. Record weight in the table on p. 8 under 0 min and your
tube number.7) Repeat steps 1-5 with the other 5 treatments.
8) Place your tube in the correct solution and start the timer (tubes 1-5 go in distilled
water in a beaker; tube 6 goes into a 40% sucrose solution). Make sure you place all the tubes into their solution and start the timer all at the same time.9) Weigh your tubes every 15 minutes for 1 hour and record the results in the table on
p. 8.10) Graph your results on the last page.
Answer the questions on p. 6-8. Work in groups initially to answer the questions on pg. 6-8, and then come together as a whole lab and report your answers to the whole lab - your TA will record these on the white board. 6Questions: Osmosis in model cells
1. Using the table below, you will first predict the results for the experiment you are going
to do today. For each experimental tube listed, predict which way water and/or sucrose will move in the table below Tube Tube contentsBeaker
contentsPrediction
1 5% sucrose Distilled
water2 10% sucrose Distilled
water3 20% sucrose Distilled
water4 40% sucrose Distilled
water5 Distilled
waterDistilled
water6 Distilled
water40% sucrose
2. What is the purpose of tubes # 5 and #6, in terms of our experiment?
3. What is happening to the concentration of water molecules and sugar molecules in the
tubes and beakers? Use the terms water potential and osmotic potential.4. What would happen to each tube if all bags were placed in 40% sucrose instead of
distilled water? (Use the table below to record your prediction) 7Tube # Tube
contentsBeaker
contentsPrediction
1 5% sucrose 40% sucrose
2 10% sucrose 40% sucrose
3 20% sucrose 40% sucrose
4 40% sucrose 40% sucrose
5 Distilled
water40% sucrose
5. To put this experiment in context, think about the literal tons of salt spread on the roads
each winter the state of Vermont in the road crew's quest to keep roads free of ice. Plants near a heavily traveled highway sometimes show signs of salt damage by the end of winter. Sometimes you can see the strip of brown plants that grow right alongside the road. This happens because passing cars spray some of the salt onto the banks and this can affect the growth of trees several meters from the road. The highest concentrations of salt are usually found in the snow banks along the side of the road. Researchers in Canada measured salt concentrations in snow banks as high as 2%. Let's consider the molarity of sodium in snow that contains 2% salt. The molecular weight of sodium chloride (NaCl) is 58.44. A 2% solution of salt would therefore be 0.34 Molar. The concentration of salt in an isotonic solution is 0.9% or 0.15 Molar. Based on your observations, are salt concentrations in the range of 2% likely to affect plant leaf cells?If so, how?
8Record your data here.
Tube TubeContents
Beaker
Contents
Weight of Bag (Grams)
0 min 15 min 30 min 45 min 60 min
1 5%Sucrose
Distilled
Water 2 10%Sucrose
Distilled
Water 3 20%Sucrose
Distilled
Water 4 40%Sucrose
Distilled
Water 5Distilled
WaterDistilled
Water 6Distilled
Water 40%Sucrose
Before leaving lab...
Turn in your drawings and complete lab assignment (all questions answered) to your TA. Keep your data on page 8 for your lab report. Points will be docked if you do not turn in your assignment before leaving lab.TOTAL: /10
9Please cite this lab manual in APA format:
Hill, L.M. and A. Clark. 2011. Osmosis in Model and Living Cells [lab manual]. PBIO 004, Introduction to Botany, University of Vermont, Burlington, VT. pp. 9. 10 11 PBIO 4 Osmosis Lab Report Rubric (for use in the coming weeks)GENERAL REQUIREMENTS (1 pt)
• Complete (title, abstract, introduction, methods, results, discussion) • Clarity of writing (how easily you can understand the ideas presented)TITLE & ABSTRACT (3 pts)
• Title describes the specific topic analyzed • Abstract is written concisely • Rationale for why the analysis was performed is given, methods are stated briefly, key results and main conclusions are describedINTRODUCTION (4 pts)
• Includes a description of the broader topic with literature cited. • Explains how the concepts of the broader question apply to the system studied • Hypothesis is clearly stated • Background information is relevant and completeMETHODS (3 pts)
• Complete description of materials and methods used to conduct Osmosis lab (put this in your own words, do not plagiarize and simply copy the lab manual) • Enough detail given that another research could repeat the analyses • Does not include irrelevant informationRESULTS (5 pts)
• Results are presented in a clear, organized fashion • Complete presentation of results of experiments involving dialysis tubing (model cell) in terms of diffusion, osmosis and water potential. • Data is presented clearly and figures are properly labeled • Figure legends are descriptive enough to understand the data presented • Results are described, but not interpretedDISCUSSION (3 pts)
• States whether the data support/do not support the hypothesis with sufficient explanation given for why the data do/do not support the hypothesis • Alternative interpretation of the data provided • Clearly describes how the results fit into the broader question being addressed with literature cited.REFERENCES (1 pt)
• At least 2 pertinent, peer-reviewed, APA cited references OVERALL ASSESSMENT OF REPORT Score:________ / 20quotesdbs_dbs14.pdfusesText_20[PDF] osmosis worksheet answer key pdf
[PDF] osu cse components api
[PDF] osu cse components binary tree
[PDF] osu cse components stack
[PDF] osu cse documentation
[PDF] oswego ny newspapers online
[PDF] osxpmem
[PDF] other names for seven deadly sins
[PDF] otis 12 gauge shotgun cleaning kit
[PDF] otpf 3rd edition pdf
[PDF] ott business model pdf
[PDF] ottawa application login
[PDF] ottawa catholic school board calendar 2019 2020
[PDF] ottawa catholic school board strike
