Communication de la Commission — Lignes directrices concernant
5 juin 2012 2. La directive relative au SEQE prévoit les mesures particulières temporaires suivantes pour certaines entreprises: des aides visant à ...
Communication de la Commission — Lignes directrices concernant
5 juin 2012 2. La directive relative au SEQE prévoit les mesures particulières temporaires suivantes pour certaines entreprises: des aides visant à ...
Université de Montréal Jean-Paul II et la communication sociale de l
Jean-Paul II a été favorable à une bonne utilisation des moyens de communication sociale pour renforcer les activités missionnaires de l'Église catholique dans
Anyidoho Paul memoire ?sequence= &isAllowed=y
Guideline on good pharmacovigilance practices (GVP) - Annex II
9 oct. 2017 Annex II – Templates: Communication Plan for Direct Healthcare Professional. Communication (CP DHPC). Draft finalised by the Agency in ...
guideline good pharmacovigilance practices gvp annex ii templates communication plan direct en
NOTICE « Solvabilité II » Communication d'informations à l'autorité
7 août 2020 NOTICE « Solvabilité II ». Communication d'informations à l'autorité de contrôle et informations à destination du public (RSR / SFCR).
. notice solvabilite rsr sfcr
Guideline on good pharmacovigilance practices (GVP) Annex II
8 déc. 2015 Annex II – Templates: Communication Plan for Direct Healthcare Professional. Communication (CP DHPC). Draft finalised by the Agency in ...
draft guideline good pharmacovigilance practices gvp annex ii templates communication plan direct en
COMMUNICATION DE LA COMMISSION Lignes directrices
La directive 2003/87/CE a été modifiée en 2018 (2) afin d'améliorer et de prolonger le SEQE de l'UE pour la période 2021-2030. 4. Le 11 décembre 2019 la
EUROPEAN COMMISSION Brussels 24.11.2021 C(2021) 8413
24 nov. 2021 Communication from the Commission of 21 September 2020 – Guidelines on ... consumption efficiency benchmarks listed in Annex II are not.
amending communication guidelines ETS annexII and III
Outils de communication II — La communication efficace … à votre
Outils de communication II Introduction. Introduction. L'entrevue médicale constitue l'outil clinique le plus important et utile au service du médecin.
H F
Logiciel de communication pour véhicules OBD-II/EOBD
Figure 2-1. Cycle de conduite typique OBD-II. Page 12. 8. INTRODUCTION. Si aucun code d'anomalie n'est signalé (concernant la panne initiale) on peut supposer
EOBD OM FR
See websites for contact details
European Medicines Agency www.ema.europa.eu
Heads of Medicines Agencies www.hma.eu
The European Medicines Agency is
an agency of the European Union © European Medicines Agency and Heads of Medicines Agencies, 2017. Reproduction is authorised provided the source is acknowledged. 9October
2017
EMA/334164/2015
Guideline on good pharmacovigilance practices (GVP)Annex II
- Templates: Communication Plan for Direct Healthcare ProfessionalCommunication (CP DHPC)
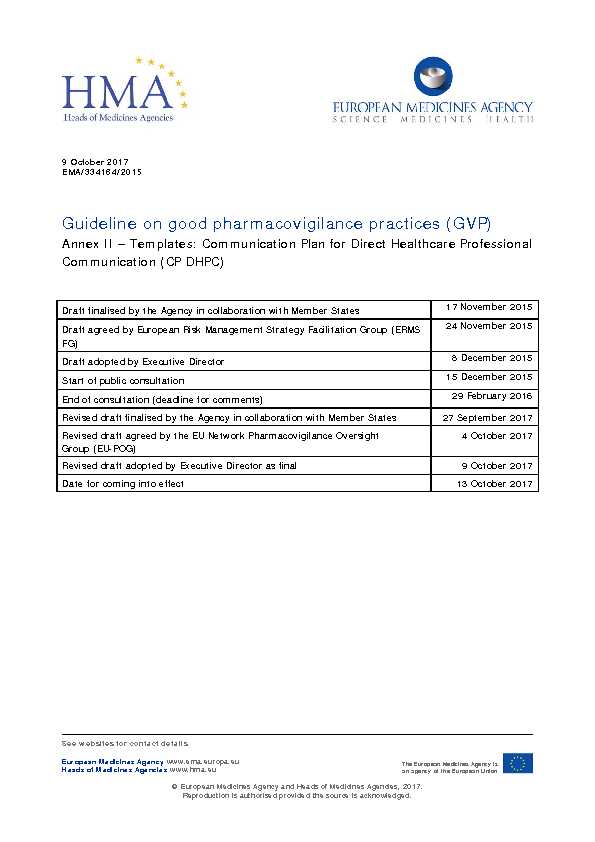
Draft finalised by the Agency in collaboration with Member States17 November 2015
Draft agreed by European Risk Management Strategy Facilitation Group (ERMS FG)24 November 2015
Draft adopted by Executive Director
8 December 2015
Start of public consultation
15 December 2015
End of consultation (deadline for comments)
29 February 2016
Revised draft finalised by the Agency in collaboration with Member States 27 September 2017 Revised draft agreed by the EU Network Pharmacovigilance Oversight
G ro up (EU-POG)4 October 2017 Revised draft adopted b
y E xecutive Director as final 9 October 2017 Date for coming into effect13 October 2017
Guideline on good pharmacovigilance practices (GVP) - Annex II - DHPC CPEMA/334164/2015 Page 2/2
DHPC COMMUNICATION PLAN
Medicinal
product(s)/active substance(s)Marketing
authorisation holder(s) In cases where the DHPC concerns several marketing authorisation holders of the same active substance or is part of a class review, it is strongly encouraged that a single consistent message is sent to healthcare professionals in each EU Member State. All concerned marketing authorisation holders in each Member State are strongly encouraged to collaborate, so that a single DHPC is prepared and circulated in each Member State. The letter circulated in eachMember State
should cover all active substance-containing products authorised in that Member Stat e. It is encouraged that the originator marketing authorisation holder (where available) in each Member State acts as the contact point for the national competent authority, on behalf of the other concerned marketing authorisation holders in the same Member State. If no originator product is marketed in the Member State, it is encouraged that one of the concerned generic companies acts as contact point for the competent authority.Safety concern and
purpose of the communicationConsider using the title of
the DHPC to describe the safety concern DHPC recipients List all (groups of) recipients of the DHPC in this section, e.g. general practitioners, specialists, community pharmacists, hospital pharmacists, nurses, professional societies, national associations.Member States where
the DHPC will be distributed Timetable Delete steps which are not applicable DateDHPC and communication plan
(in English) agreed by PRACDHPC and communication plan
(in English) agreed by CHMP/CMDh Submission of translated DHPCs to the national competent authorities for reviewAgreement of translations by
national competent authoritiesDissemination of DHPC
See websites for contact details
European Medicines Agency www.ema.europa.eu
Heads of Medicines Agencies www.hma.eu
The European Medicines Agency is
an agency of the European Union © European Medicines Agency and Heads of Medicines Agencies, 2017. Reproduction is authorised provided the source is acknowledged. 9October
2017
EMA/334164/2015
Guideline on good pharmacovigilance practices (GVP)Annex II
- Templates: Communication Plan for Direct Healthcare ProfessionalCommunication (CP DHPC)
Draft finalised by the Agency in collaboration with Member States17 November 2015
Draft agreed by European Risk Management Strategy Facilitation Group (ERMS FG)24 November 2015
Draft adopted by Executive Director
8 December 2015
Start of public consultation
15 December 2015
End of consultation (deadline for comments)
29 February 2016
Revised draft finalised by the Agency in collaboration with Member States 27 September 2017 Revised draft agreed by the EU Network Pharmacovigilance Oversight
G ro up (EU-POG)4 October 2017 Revised draft adopted b
y E xecutive Director as final 9 October 2017 Date for coming into effect13 October 2017
Guideline on good pharmacovigilance practices (GVP) - Annex II - DHPC CPEMA/334164/2015 Page 2/2
DHPC COMMUNICATION PLAN
Medicinal
product(s)/active substance(s)Marketing
authorisation holder(s) In cases where the DHPC concerns several marketing authorisation holders of the same active substance or is part of a class review, it is strongly encouraged that a single consistent message is sent to healthcare professionals in each EU Member State. All concerned marketing authorisation holders in each Member State are strongly encouraged to collaborate, so that a single DHPC is prepared and circulated in each Member State. The letter circulated in eachMember State
should cover all active substance-containing products authorised in that Member Stat e. It is encouraged that the originator marketing authorisation holder (where available) in each Member State acts as the contact point for the national competent authority, on behalf of the other concerned marketing authorisation holders in the same Member State. If no originator product is marketed in the Member State, it is encouraged that one of the concerned generic companies acts as contact point for the competent authority.Safety concern and
purpose of the communicationConsider using the title of
the DHPC to describe the safety concern DHPC recipients List all (groups of) recipients of the DHPC in this section, e.g. general practitioners, specialists, community pharmacists, hospital pharmacists, nurses, professional societies, national associations.Member States where
the DHPC will be distributed Timetable Delete steps which are not applicable DateDHPC and communication plan
(in English) agreed by PRACDHPC and communication plan
(in English) agreed by CHMP/CMDh Submission of translated DHPCs to the national competent authorities for reviewAgreement of translations by
national competent authoritiesDissemination of DHPC
- iti communication
- iti communications llc
- iti communication societe.com
- iti communication limoges recrutement
- iti communication nevers
- iti communications zoominfo
- scott bartlett iti communications
- iti information communication technology system maintenance jobs