 URGENT MEDICAL DEVICE RECALL
URGENT MEDICAL DEVICE RECALL
16 апр. 2020 г. BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF (Sterile ... Please assist BD with assuring these ...
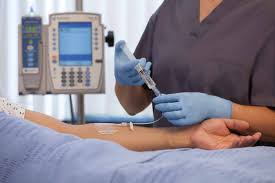 BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
to help reduce complications and improve patient care. BD VAM assessments establish the baseline 5 mL BD PosiFlush™ Heparin Lock Flush Syringe 50 usp units/5 ...
 BD PosiFlushTM Pre-Filled Flush Syringes
BD PosiFlushTM Pre-Filled Flush Syringes
BD PosiFlush™ helps reduce the risk of catheter related bloodstream BD Medical. The Danby Building. Edmund Halley Road. Oxford Science Park. OXFORD OX4 4DQ.
 BD PosiFlush™
BD PosiFlush™
BD Luer-Lok™ Tip Cap: Tighter seal for closure integrity helping prevent touch contamination. Clear labelling helps reduce the risk of medication errors.
 BD PosiFlush™ Prefilled Saline Syringe Supply Update
BD PosiFlush™ Prefilled Saline Syringe Supply Update
Dear BD Valued Customer. We would like to provide you with confidence that BD is working diligently to support your prefilled flush syringe needs through our
 BD Medical Medical Surgical Systems Catalogue Canadian Version
BD Medical Medical Surgical Systems Catalogue Canadian Version
BD Activation-Assist™ technology for fast and easy needle tip shielding more than twice as many BD PosiFlush 3 mL size syringes into a 5.1 L In-Room ...
 BD Medical – Recall of heparin lock flush and normal saline syringes
BD Medical – Recall of heparin lock flush and normal saline syringes
20 апр. 2018 г. The BD PosiFlush heparin lock flush syringe is intended to help maintain patency by locking vascular access devices. • The BD pre-filled ...
 Think BD PosiFlush™ Syringes filled with Saline.
Think BD PosiFlush™ Syringes filled with Saline.
BD PosiFlush™ SP Syringes are not intended for use on a sterile field. Page 3. Silicone Lubricant. Medical-grade silicone oil is applied to the stopper and
 TABLE OF CONTENTS
TABLE OF CONTENTS
2 февр. 2017 г. The 0.9% Sodium Chloride Injection USP
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 Apr 2018 bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ HEPARIN LOCK FLUSH ... in Attachment B to aid you with the identification of the.
 BD PosiFlush Recall
BD PosiFlush Recall
16 Apr 2020 BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF. (Sterile Field) Saline Flush Syringe 10mL ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ PRE-FILLED NORMAL SALINE SYRINGE If you require further assistance please contact: Contact.
 URGENT MEDICAL DEVICE RECALL
URGENT MEDICAL DEVICE RECALL
16 Apr 2020 BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF. (Sterile Field) Saline Flush Syringe 10mL ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 Apr 2018 bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ HEPARIN LOCK FLUSH ... in Attachment B to aid you with the identification of the.
 BD PosiFlushTM - Because so much is on the line
BD PosiFlushTM - Because so much is on the line
The BD PosiFlush™ Prefilled Saline Syringe is a solution that is part of the BD Organisation for Standardisation (ISO) 13485 standard on Medical.
 Think BD PosiFlush™ Syringes filled with Saline.
Think BD PosiFlush™ Syringes filled with Saline.
BD PosiFlush™ SP Syringes filled with Saline
 BD Medical – Recall of heparin lock flush and normal saline syringes
BD Medical – Recall of heparin lock flush and normal saline syringes
20 Apr 2018 The BD PosiFlush heparin lock flush syringe is intended to help maintain patency by locking vascular access devices. • The BD pre-filled normal ...
 AUG 2 6 2004 000016
AUG 2 6 2004 000016
6 Feb 2004 USP BD Posiflush SF Pre-Filled Flush Syringe was modified by changing the current ... 1976 the enactment date of the Medical Device.
 BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
to provide clinical benefits to help improve patient care manufacture over 14 billion BD PosiFlush™ Sterile Path Syringes. Saline and syringes.
MPS-18-1248-FA Page 1 of 9
BD Medical
1 Becton Drive
Franklin Lakes, NJ 07417
USA bd.comURGENT MEDICAL DEVICE RECALL
BD POSIFLUSH +(3$5H1 I2F. )I86+ 6K5H1*(6
%G 35(-FILLED NORMAL SALINE SYRINGES April20, 2018
Product Name Catalog (REF) Number BD PosiFlushLock Flush Syringes 306509, 306510, 306511, 306512, 306513,
306514, 306515, 306516, 306517, 306521,
306525, 306528, 306531 -Filled Normal Saline Syringes 306500, 306502, 306503, 306504, 306505,
306507, 306508, 306518
For the Attention of:
Risk Manager, Materials Manager, Infection Control, Medical Director, Medical Device Safety Officer, Director of Nursing, Nursing Education, Pharmacy DirectorsDescription of the problem and health hazard(s):
Out of an abundance of caution and in the interest of public health, BD is voluntarily recalling certain
lots of -Filled Normal Saline Flush syringes due to a potential for contamination with Serratia marcescens bacterium. BD was notified by the U.S. Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) about apotential epidemiological link between catheter related blood stream infections and the S. marcescens
bacterium.Specifically, the FDA and CDC identified a potential connection between reports of infection in a small
number of patients caused by S. marcescens across multiple that affected patients had received treatment using certain BD flush products.To date, there is no evidence of BD flush product testing positive for this bacterium. Investigations are
ongoing by BD, FDA, and CDC.This recall only affects the product Catalog (REF) and lot numbers listed in Attachment A: List of Recall
Catalog Numbers and Lot Numbers, all from a single manufacturing site. No products other than those listed in the Attachment A are affected by this recall.Examples of the product labels are provided in Attachment B to aid you with the identification of the
affected product.MPS-18-1248-FA Page 2 of 9
BD Medical
1 Becton Drive
Franklin Lakes, NJ 07417
USA bd.comYOU NEED TO TAKE THE FOLLOWING ACTIONS:
1. Immediately review your inventory for the specific Catalog (Ref) and Lot numbers listed in
Attachment A, discontinue use and quarantine product subject to the recall.2. Complete the Customer Recall Response Form and fax it to BD at 1-855-620-5693 or email to
BD5689@stericycle.com. Even if you do not have any of the affected lot(s) in your inventory, please complete the Customer Recall Response Form indicating you have zero (0) quantity andreturn as indicated above. 3. If you have inventory of the recalled product, please return the product following the enclosed
packing instructions. This is required so that BD may process your product replacement upon receipt of the recalled product.4. Share this notice with all users of the product within your facility to ensure awareness.
Contact Information
If you have questions or require further assistance, please contact 1-866-660-8973 between 8 AM and 5 PM ET Monday through Friday. Any adverse health consequences experienced with the use of this product should be reported to BD and may be reported to the FDA's MedWatch Adverse Event Reporting program.Web: MedWatch website at www.fda.gov/medwatch
Phone: 1-800-FDA-1088 (1-800-332-1088)
Mail: MedWatch, HF--9787
This recall is being conducted with the knowledge of the FDA. Patient safety and product quality are
top priorities at BD, and the company takes any potential product issue very seriously. Our primaryobjective is to provide you with quality products that are safe for patients and users. We thank you in
advance for complying with this medical device recall notification as quickly and effectively as possible.
Sincerely,
Klaus Hoerauf, MD Gail Griffiths
VP Global Medical Affairs Sr. Director Corporate Regulatory ComplianceBD Medication Delivery Solutions BD US Region
MPS-18-1248-FA Page 3 of 9
BD Medical
1 Becton Drive
Franklin Lakes, NJ 07417
USA bd.comAttachment A
List of Recall Catalog (REF) and Lot Numbers
Product Name UDI Catalog
(Ref)No. First Lot
Number
Affected Last Lot
Number
Affected Product Package Size
-Filled Normal Saline Syringe (01)50382903065009 306500 708111D 735212A 120 units per case (30 units per shelf box) -Filled Normal Saline Syringe (01)50382903065023 306502 706012B 734511C 120 units per case (30 units per shelf box) -Filled Normal Saline Syringe (01)50382903065030 306503 706512B 730611C 120 units per case (30 units per shelf box) -Filled Normal Saline Syringe (01)50382903065047 306504 714312C 735212C 120 units per case (30 units per shelf box) -Filled Normal Saline Syringe (01)50382903065054 306505 714511C 730411C 120 units per case (30 units per shelf box) -Filled Normal Saline Syringe (01)50382903065078 306507 705311B 735211C 120 units per case (30 units per shelf box) -Filled Normal Saline Syringe (01)50382903065085 306508 706211B 734211C 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065092 306509 710272N 734111N 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065108 306510 707672N 735222N 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065115 306511 707671N 735221N 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065122 306512 710271N 733811N 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065139 306513 707471N 735211N 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065146 306514 708782N 734211N 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065153 306515 707971N 735311N 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065160 306516 708071N 730612N 120 units per case (30 units per shelf box)MPS-18-1248-FA Page 4 of 9
BD Medical
1 Becton Drive
Franklin Lakes, NJ 07417
USA bd.comProduct Name UDI Catalog
(Ref)No. First Lot
Number
Affected Last Lot
Number
Affected Product Package Size
Lock Flush Syringe (01)50382903065177 306517 710371N 734221N 120 units per case (30 units per shelf box) -Filled NormalSaline Syringe with Blunt
Plastic Cannula (01)50382903065184 306518 715013C 735211A 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065214 306521 710071N 731921N 120 units per case (30 units per shelf box)Lock Flush Syringe with
Blunt Plastic Cannula (01)50382903065252 306525 713772N 734011N 120 units per case (30 units per shelf box) Lock Flush Syringe (01)50382903065283 306528 710372N 719121N 120 units per case (30 units per shelf box)Lock Flush Syringe with
Blunt Plastic Cannula (01)50382903065313 306531 716091N 733421N 120 units per case (30 units per shelf box)MPS-18-1248-FA Page 5 of 9
BD Medical
1 Becton Drive
Franklin Lakes, NJ 07417
USA bd.comAttachment B
Medical Device Recall Catalog (Ref) and Lot Numbers Identification Sample: Example for Saline: Case Label:
Shelf Box Label: Unit Label: Catalog (REF) NumberCatalog (REF) Number
Catalog (REF) Number Lot Number
Lot Number
Lot Number
MPS-18-1248-FA Page 6 of 9
BD Medical
1 Becton Drive
Franklin Lakes, NJ 07417
USA bd.comExample for 10 unit Heparin:
Case Label:
Shelf Box Label:
Unit Label:
Catalog (REF) Number
Catalog (REF) Number
Catalog (REF) Number Lot Number
Lot Number
Lot Number
MPS-18-1248-FA Page 7 of 9
BD Medical
1 Becton Drive
Franklin Lakes, NJ 07417
USA bd.comExample for 100 unit Heparin:
Case Label:
Shelf Box Label:
Unit Label:
Catalog (REF) Number
Catalog (REF) Number
Catalog (REF) Number Lot Number Lot Number
Lot Number
MPS-18-1248-FA Page 8 of 9
BD Medical
1 Becton Drive
Franklin Lakes, NJ 07417
USA bd.comCustomer Recall Response Form
BD PosiFlush
BD-Filled Normal Saline Syringes
Please assist BD by promptly returning this form to: BDEmail: BD5689@stericycle.com
Fax No.: 1-855-620-5693
Facility: Please use full, current facility name. Do not use initials. Street Address:
City: State: Zip: ______
Contact Person:
Telephone No.:
Email Address:
Fax No.: ______________________
Name:Title:
Signature/Date:
I have read and understood the contents of this product recall notification and confirm that ourproduct inventory has been checked. Please select one of the following: We do not have any of the affected product(s) on hand. We have the following affected product in our inventory:
Product Name Catalog No. Lot No. No. of Units
I certify that I have returned all affected product indicated above as available inventory at the time
of receipt of this notification.MPS-18-1248-FA Page 9 of 9
BD Medical
1 Becton Drive
Franklin Lakes, NJ 07417
USA bd.comPACKING INSTRUCTIONS
Urgent Medical Device Recall
Product Return Instructions:
1. Please enclose the completed Customer Recall Response Form with the shipment.
2. The simplest way to return product would be to access the following UPS website:
http://returns.upsrow.comLogin ID: bdapi, Password: bdapi
When you access the site, you can select among 4 UPS options. If you select the options,person who stops at your site or drop it off at a UPS location. If you select either of the remaining
two options, a UPS person will stop by your location specifically to pick up the package. You need to enter the returned product reorder number, lot number and quantity on the website. Note: If you are not returning product, also indicate this on the website.3. If you do not have access to the internet you can call UPS at 1-800-PICK-UPS (742-5877) and
arrange for a pick-up using the following charge number specific to this recall: 0ER739.Product should be returned to:
Returns Team
BD Distribution Center
Door #2
130 Four Oaks Parkway
Four Oaks, NC 27524
For shipments over 150 pounds - utilize UPS Ground Freight. UPS Freight Customer Service can be contacted at 1-800-333-7400. When arranging the pick-up of freight, please specify 3rd party billing as follows:Returns Team
BD c/o Cass Info Systems
PO Box 67
St. Louis, MO 63166-0067
4. Upon receipt of returned product BD will issue a product replacement. A returned goods
authorization is NOT required for this recall return process.DO NOT SHIP FREIGHT COLLECT
2XU RMUHORXVH ŃMQQRP UHŃHLYH SURGXŃPV VOLSSHG ³IUHLJOP ŃROOHŃP´B
quotesdbs_dbs26.pdfusesText_32[PDF] BD Tryptic Soy Broth (TSB)
[PDF] BD-FIL 2014 - Horaires des dédicaces du vendredi 12 septembre - Festival
[PDF] BD-jeunesse_2015-04
[PDF] BD-S1065_FICHE:Mise en page 1
[PDF] Bd. 72 1972 Quellen und Forschungen aus italienischen
[PDF] BDAF120_Decembre2003
[PDF] BDC - Anciens Et Réunions
[PDF] BDC 2014 - Amitié Solidarité Savoie - France
[PDF] bdc 2015 - CE Legrand Lagord - Anciens Et Réunions
[PDF] bdc AGRO - Conception
[PDF] BDC Beaux-Livres réassort - Anciens Et Réunions
[PDF] BDC CIVILITE 2014
[PDF] BDC FDE - Anciens Et Réunions
[PDF] bdc flammes pub - Logiciels Graphiques
