 URGENT MEDICAL DEVICE RECALL
URGENT MEDICAL DEVICE RECALL
16 апр. 2020 г. BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF (Sterile ... Please assist BD with assuring these ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 апр. 2018 г. BD PosiFlush™ Heparin Lock Flush Syringes. 306509 306510
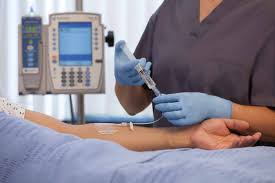 BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
to help reduce complications and improve patient care. BD VAM assessments establish the baseline 5 mL BD PosiFlush™ Heparin Lock Flush Syringe 50 usp units/5 ...
 BD PosiFlushTM Pre-Filled Flush Syringes
BD PosiFlushTM Pre-Filled Flush Syringes
BD PosiFlush™ helps reduce the risk of catheter related bloodstream BD Medical. The Danby Building. Edmund Halley Road. Oxford Science Park. OXFORD OX4 4DQ.
 BD PosiFlush™
BD PosiFlush™
BD Luer-Lok™ Tip Cap: Tighter seal for closure integrity helping prevent touch contamination. Clear labelling helps reduce the risk of medication errors.
 BD PosiFlush™ Prefilled Saline Syringe Supply Update
BD PosiFlush™ Prefilled Saline Syringe Supply Update
Dear BD Valued Customer. We would like to provide you with confidence that BD is working diligently to support your prefilled flush syringe needs through our
 BD Medical Medical Surgical Systems Catalogue Canadian Version
BD Medical Medical Surgical Systems Catalogue Canadian Version
BD Activation-Assist™ technology for fast and easy needle tip shielding more than twice as many BD PosiFlush 3 mL size syringes into a 5.1 L In-Room ...
 BD Medical – Recall of heparin lock flush and normal saline syringes
BD Medical – Recall of heparin lock flush and normal saline syringes
20 апр. 2018 г. The BD PosiFlush heparin lock flush syringe is intended to help maintain patency by locking vascular access devices. • The BD pre-filled ...
 Think BD PosiFlush™ Syringes filled with Saline.
Think BD PosiFlush™ Syringes filled with Saline.
BD PosiFlush™ SP Syringes are not intended for use on a sterile field. Page 3. Silicone Lubricant. Medical-grade silicone oil is applied to the stopper and
 TABLE OF CONTENTS
TABLE OF CONTENTS
2 февр. 2017 г. The 0.9% Sodium Chloride Injection USP
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 Apr 2018 bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ HEPARIN LOCK FLUSH ... in Attachment B to aid you with the identification of the.
 BD PosiFlush Recall
BD PosiFlush Recall
16 Apr 2020 BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF. (Sterile Field) Saline Flush Syringe 10mL ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ PRE-FILLED NORMAL SALINE SYRINGE If you require further assistance please contact: Contact.
 URGENT MEDICAL DEVICE RECALL
URGENT MEDICAL DEVICE RECALL
16 Apr 2020 BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF. (Sterile Field) Saline Flush Syringe 10mL ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 Apr 2018 bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ HEPARIN LOCK FLUSH ... in Attachment B to aid you with the identification of the.
 BD PosiFlushTM - Because so much is on the line
BD PosiFlushTM - Because so much is on the line
The BD PosiFlush™ Prefilled Saline Syringe is a solution that is part of the BD Organisation for Standardisation (ISO) 13485 standard on Medical.
 Think BD PosiFlush™ Syringes filled with Saline.
Think BD PosiFlush™ Syringes filled with Saline.
BD PosiFlush™ SP Syringes filled with Saline
 BD Medical – Recall of heparin lock flush and normal saline syringes
BD Medical – Recall of heparin lock flush and normal saline syringes
20 Apr 2018 The BD PosiFlush heparin lock flush syringe is intended to help maintain patency by locking vascular access devices. • The BD pre-filled normal ...
 AUG 2 6 2004 000016
AUG 2 6 2004 000016
6 Feb 2004 USP BD Posiflush SF Pre-Filled Flush Syringe was modified by changing the current ... 1976 the enactment date of the Medical Device.
 BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
to provide clinical benefits to help improve patient care manufacture over 14 billion BD PosiFlush™ Sterile Path Syringes. Saline and syringes.
BD PosiFlush
TM - Because so much is on the line Step inside our manufacturing plant to experience the quality ofBD PosiFlush
TMPrefilled Saline Syringes
IV catheter-related infections -
patients at riskBD PosiFlush
TM - focusing on product quality, enhancing patient safetyDid you know?Step inside the BD plant in
Fraga, Spain
60-90% of all
patients admitted to hospital receive a vascular access device 2 Approximately 80% of all hospital patients receive IV therapy 1 and it is the most common way to administer medication in healthcare facilities. 2 However, IV catheter failure may cause pain, dissatisfaction, extended length of care and vein depletion. 2The BD PosiFlush™ Prefilled Saline Syringe is a solution that is part of the BD vascular access management portfolio.
The polypropylene syringe contains a sterile 0.9% sodium chloride solution used to flush in-situ vascular access devices.
This may minimise the risk of IV complications such as catheter contamination. 4A "hands free" process - ensuring
quality at each step From raw material to finished syringe, there is no human contact, to maximise product safetyRegular inspections throughout the process
Chemical and biological laboratory testing
Each and every syringe is inspectedAround
40% of
healthcare-associated bloodstream infections in Europe are catheter related 2The additional cost of
catheter-related infections per patient in the UnitedKingdom is estimated at
£2,949-£6,209
(€4,392-€9,251) 3Manufacturing
quality for patient safetyQuality from production line to IV line
Our ownership of saline compounding and syringe
production ensures quality and consistency in supply. Using a fully automated production process from start to finish limits interruptions in production due to lapses in the supply chain, and allows us to have control over all steps involved in making pre-filled syringes, such as: • Quality of raw material • Optimised syringe design for flushing • Extended shelf-life • Sterile saline solutionThe BD PosiFlush
TM team follows the International Organisation for Standardisation (ISO) 13485 standard on Medical devices - Quality management systems - Requirements for regulatory purposes.What makes BD PosiFlush
TMSyringes different
BD PosiFlush
TMSyringes are designed and manufactured to
help clinicians deliver quality care to patientsBD Luer-Lok
TMTip Cap
Luer lok tip cap ensures closure
integrity for the 3 years shelf life, helping prevent touch contamination and infectionCarton shelf pack
Easy-to-open packaging with
individually wrapped syringes in an internal box, simplifying inventory management and allowing for convenient placement on shelvesClear labeling
Supports easy identification of
syringe volume, lot number and expiration date "We make the best product possible, so that healthcare workers can focus on the patient." Alberto Villalba, BD PosiFlush™ production manager "Quality is a matter of trust."Javier Pardiño, BD Fraga plant director
The largest medical device
sterilisation centre in EuropeThe Fraga plant is the largest medical device
sterilisation centre in Europe. 5BD PosiFlush™
Prefilled Saline Syringes are sterilised using moist heat, which eliminates microorganisms. 6Tests are performed at the end of each production
cycle to ensure each syringe contains the least amount of defects possible and is ready to be used for e?ective patient care.BD PosiFlush
TM5mL Syringe
Patented, unique
stubby design that reduces PSI forceBD offers a comprehensive flush portfolio to meet your patients' needsSame syringe diameter regardless of volume
BD PosiFlush™ Prefilled Saline Syringes come in three volumes (3 mL, 5 mL and 10 mL) of equal diameter (10 mL syringe).
Because the BD PosiFlush™ 3-mL Syringe has the same diameter as a standard 10-mL syringe, it generates less pressure
than smaller diameter syringes and reduces the risk of catheter damage. 7BD PosiFlush
TM ?-mL SyringeBD PosiFlush TMSP Prefilled Saline Syringes
Catheter type and size, medication as well as some of the patient characteristics should determine the flush volume being used in order to properly maintain catheter patency and reduce medication interactions. 8Standard ?-mL syringeBD PosiFlush
TMXS Prefilled Saline Syringes
This syringe is terminally sterilised in its peel pouch, enabling it to be aseptically presented to a sterile field.19.75 psi**55 psi**
* As of March 2020 ** pounds per square inch bd.com BD, the BD Logo, BD Luer-Lok and BD PosiFlush are trademarks of Becton, Dickinson and Company. ©2020 BD and its affiliates. All rights reserved. 0000CF04510 Issue 1 BD Switzerland Sàrl, Terre Bonne Park - A4, Route de Crassier 17, 1262 Eysins, Switzerland Discover BD Patient Safety: eu.bd.com/patient-safety1. Doyle GR, McCutcheon JA. Clinical Procedures for Safer Patient Care. British Columbia, CA: British Columbia Ministry of Advanced Education; 2012.
2. Helm RE, Klausner JD, Klemperer JD, Flint LM, Huang E. Accepted but unacceptable: peripheral IV catheter failure.
J Infus Nurs.
2015;38(3):189-203.
3.Tacconelli E, Smith G, Hieke K et al. Epidemiology, medical outcomes and costs of catheter-related bloodstream infections in intensive care units of four European countries: literatureand
registry-based estimates. Journal of Hospital Infection. 2009;72(2):97-103.4. Sacha G, Rogers JA, Miller RL. Pre-filled syringes: a review of the history, manufacturing and challenges.
Pharm Dev Technol.
2015;20(1):1-11. doi: 10.3109/10837450.2014.982825.
5.Europa Press. La empresa Becton, Dickinson and Company, de Fraga, logra el Premio Empresa Huesca 2011. 20 minutos. 24 March 2011. Accessed on 19 November 2019, at
6.Brown K, Graf LM, Guyader MJ, et al. Technical Report No. 48 Moist Heat Sterilizer Systems: Design, Commissioning, Operation, Qualification and Maintenance. Bethesda, Maryland,
U nited States: Parenteral Drug Association; 2010. 7.Start K. Prefilled saline flushes. Hospital Pharmacy Europe. Published on 18 August 2010. Accessed on 25 November 2019, at https://hospitalpharmacyeurope.com/news/editors-pick/
prefilled-saline-flushes/8. Gorski, LA. The 2016 Infusion Therapy Standards of Practice. Home Healthcare Now. 2017;35(1):10-18.
Prepare
Secure
ConnectMaintainSelect
Vascular Access Management
service programme Placequotesdbs_dbs26.pdfusesText_32[PDF] BD Tryptic Soy Broth (TSB)
[PDF] BD-FIL 2014 - Horaires des dédicaces du vendredi 12 septembre - Festival
[PDF] BD-jeunesse_2015-04
[PDF] BD-S1065_FICHE:Mise en page 1
[PDF] Bd. 72 1972 Quellen und Forschungen aus italienischen
[PDF] BDAF120_Decembre2003
[PDF] BDC - Anciens Et Réunions
[PDF] BDC 2014 - Amitié Solidarité Savoie - France
[PDF] bdc 2015 - CE Legrand Lagord - Anciens Et Réunions
[PDF] bdc AGRO - Conception
[PDF] BDC Beaux-Livres réassort - Anciens Et Réunions
[PDF] BDC CIVILITE 2014
[PDF] BDC FDE - Anciens Et Réunions
[PDF] bdc flammes pub - Logiciels Graphiques
