 URGENT MEDICAL DEVICE RECALL
URGENT MEDICAL DEVICE RECALL
16 апр. 2020 г. BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF (Sterile ... Please assist BD with assuring these ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 апр. 2018 г. BD PosiFlush™ Heparin Lock Flush Syringes. 306509 306510
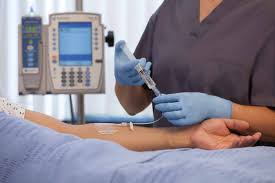 BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
to help reduce complications and improve patient care. BD VAM assessments establish the baseline 5 mL BD PosiFlush™ Heparin Lock Flush Syringe 50 usp units/5 ...
 BD PosiFlushTM Pre-Filled Flush Syringes
BD PosiFlushTM Pre-Filled Flush Syringes
BD PosiFlush™ helps reduce the risk of catheter related bloodstream BD Medical. The Danby Building. Edmund Halley Road. Oxford Science Park. OXFORD OX4 4DQ.
 BD PosiFlush™
BD PosiFlush™
BD Luer-Lok™ Tip Cap: Tighter seal for closure integrity helping prevent touch contamination. Clear labelling helps reduce the risk of medication errors.
 BD PosiFlush™ Prefilled Saline Syringe Supply Update
BD PosiFlush™ Prefilled Saline Syringe Supply Update
Dear BD Valued Customer. We would like to provide you with confidence that BD is working diligently to support your prefilled flush syringe needs through our
 BD Medical Medical Surgical Systems Catalogue Canadian Version
BD Medical Medical Surgical Systems Catalogue Canadian Version
BD Activation-Assist™ technology for fast and easy needle tip shielding more than twice as many BD PosiFlush 3 mL size syringes into a 5.1 L In-Room ...
 BD Medical – Recall of heparin lock flush and normal saline syringes
BD Medical – Recall of heparin lock flush and normal saline syringes
20 апр. 2018 г. The BD PosiFlush heparin lock flush syringe is intended to help maintain patency by locking vascular access devices. • The BD pre-filled ...
 Think BD PosiFlush™ Syringes filled with Saline.
Think BD PosiFlush™ Syringes filled with Saline.
BD PosiFlush™ SP Syringes are not intended for use on a sterile field. Page 3. Silicone Lubricant. Medical-grade silicone oil is applied to the stopper and
 TABLE OF CONTENTS
TABLE OF CONTENTS
2 февр. 2017 г. The 0.9% Sodium Chloride Injection USP
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 Apr 2018 bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ HEPARIN LOCK FLUSH ... in Attachment B to aid you with the identification of the.
 BD PosiFlush Recall
BD PosiFlush Recall
16 Apr 2020 BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF. (Sterile Field) Saline Flush Syringe 10mL ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ PRE-FILLED NORMAL SALINE SYRINGE If you require further assistance please contact: Contact.
 URGENT MEDICAL DEVICE RECALL
URGENT MEDICAL DEVICE RECALL
16 Apr 2020 BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF. (Sterile Field) Saline Flush Syringe 10mL ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 Apr 2018 bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ HEPARIN LOCK FLUSH ... in Attachment B to aid you with the identification of the.
 BD PosiFlushTM - Because so much is on the line
BD PosiFlushTM - Because so much is on the line
The BD PosiFlush™ Prefilled Saline Syringe is a solution that is part of the BD Organisation for Standardisation (ISO) 13485 standard on Medical.
 Think BD PosiFlush™ Syringes filled with Saline.
Think BD PosiFlush™ Syringes filled with Saline.
BD PosiFlush™ SP Syringes filled with Saline
 BD Medical – Recall of heparin lock flush and normal saline syringes
BD Medical – Recall of heparin lock flush and normal saline syringes
20 Apr 2018 The BD PosiFlush heparin lock flush syringe is intended to help maintain patency by locking vascular access devices. • The BD pre-filled normal ...
 AUG 2 6 2004 000016
AUG 2 6 2004 000016
6 Feb 2004 USP BD Posiflush SF Pre-Filled Flush Syringe was modified by changing the current ... 1976 the enactment date of the Medical Device.
 BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
to provide clinical benefits to help improve patient care manufacture over 14 billion BD PosiFlush™ Sterile Path Syringes. Saline and syringes.
AUG 2 6 2004
510K SUMMARY OF SAFETY AND EFFECTIVENESS
1. Submitted By:
Peter Zurlo
Manager, Regulatory Affairs
B D Medical Surgical
I Becton Drive
Franklin Lakes, NJ 07417-1883
Phone: 201-847-6447
Fax: 201-847-4855
2. Device Name:
Trade Name: 0.9% Sodium Chloride Injection, USP-
BD Posiflush SF Flush Syringe
Common Name: Saline Flush Syringe
Classification Name: Device, Flush, Vascular Access3. Predicate Device:
0.9% Sodium Chloride Injection, USP-
BD Posiflush SP Pre-filled Flush Syringe
Manufactured by: Becton Dickinson and Company
4. Device Description:
The Predicate Device, the 0.9% Sodium Chloride Injection, USP- BD Posiflush SP Pre- Filled Flush Syringe (510(k) Number: K003553) is a single use disposable Hypodermic syringe filled with 0.9% Sodium Chloride Injection, USP and is intended for use in maintaining patency of vascular access devices (VAD's). The Predicate Device is only fluid path sterile to SAL of 10-6. The Modified Device, the subject of this 510(k), the 0.9% Sodium Chloride Injection, USP BD Posiflush SF Pre-Filled Flush Syringe was modified by changing the current individual package wrap to a package that is capable of being sterilized and will maintain sterility of the exterior of the device. This will allow the device to be used on sterile field applications. The Modified Device is manufactured of the same materials, has the same intended use and SAL of 106 as the Predicate Device.
000016
5. Intended Use:
Same intended use as the Predicate Device.
The 0.9% Sodium Chloride Injection, USP, BD Posiflush SF Flush Syringes is intended for use in maintaining patency of vascular access devices (VAD's).Technological Characteristics:
The Modified Device, the subject of this 510(k), the 0.9% Sodium Chloride Injection, USP BD Posiflush SF Pre-Filled Flush Syringe was modified by changing the current individual package wrap to a package that is capable of being sterilized and will maintain sterility of the exterior of the device. This will allow the device to be used on sterile field applications. The Modified Device is manufactured of the same materials, has the same intended use and SAL of 106 as the Predicate Device.
6. Performance:
Design Verification tests were performed based on the risk analysis performed and the results of these tests demonstrate that the BD Posiflush SF Flush Syringe performed in an equivalent manner to the predicate device and is safe and effective when used as intended. The term "substantial equivalence" as used in this 510(k) notification is limited to the definition of substantial equivalence found in the Federal Food, Drug, and Cosmetic Act, as amended and as applied under 21 CFR 807, Subpart E, under which a device can be marketed without pre-approval or classification. A determination of substantial equivalency under this notification is not intended to have any bearing whatsoever on the resolution of patent infringement suits or any other patent matters. No statements related to, or in support of substantial equivalence herein shall be construed as an admission against interest under the US patent Laws or their application by the courts.00001:7
DEPARTMENT OF HEALTH & HUMAN SERVICES
Public Health Service
Food and Drug Administration
9200 Corporate Boulevard
Rockville MD 20850
AUG 2 6 2004
Becton Dickinson
CIO Mr. Peter Zurlo
Manager, Regulatory Affairs
BD Medical Surgical
1 Becton Drive
Franklin Lakes, New Jersey 07417
Re: K042061
Trade/Device Name: 0.9% Sodium Chloride Injection, USP, BD Posiflush SF FlushSyringe
Regulation Number: 880.5200
Regulation Name: Intravascular Catheter
Regulatory Class: II
Product Code: NGT
Dated: July 29, 2004
Received: August 2, 2004
Dear Mr. Zurlo:
We have reviewed your Section 510(k) premarket notification of intent to market the device referenced above and have determined the device is substantially equivalent (for the indications for use stated in the enclosure) to legally marketed predicate devices marketed in interstate commerce prior to May 28, 1976, the enactment date of the Medical Device Amendments, or to devices that have been reclassified in accordance with the provisions of the Federal Food, Drug, and Cosmetic Act (Act) that do not require approval of a premarket approval application (PMA). You may, therefore, market the device, subject to the general controls provisions of the Act. The general controls provisions of the Act include requirements for annual registration, listing of devices, good manufacturing practice, labeling, and prohibitions against misbranding and adulteration. If your device is classified (see above) into either class 1 (Special Controls) or class III (PMA), it may be subject to such additional controls. Existing major regulations affecting your device can be found in the Code of Federal Regulations, Title 21, Parts 800 to 898. In addition, FDA may publish further announcements concerning your device in the FederalRegister.
Page 2 -Mr. Zurlo
Please be advised that FDA's issuance of a substantial equivalence determination does not mean that FDA has made a determination that your device complies with other requirements of the Act or any Federal statutes and regulations administered by other Federal agencies. You must comply with all the Act's requirements, including, but not limited to: registration and listing (21 CFR Part 807); labeling (21 CFR Part 801); good manufacturing practice requirements as set forth in the quality systems (QS) regulation (21 CFR Part 820); and if applicable, the electronic product radiation control provisions (Sections 531-542 of the Act);21 CFR 1000-1050.
This letter will allow you to begin marketing your device as described in your Section 510(k) premarket notification. The FDA finding of substantial equivalence of your device to a legally marketed predicate device results in a classification for your device and thus, permits your device to proceed to the market. If you desire specific advice for your device on our labeling regulation (21 CFR Part 801), please contact the Office of Compliance at (301) 594-4618. Also, please note the regulation entitled, "Misbranding by reference to premarket notification" (21CFR Part 807.97). You may obtain other general information on your responsibilities under the Act from the Division of Small Manufacturers, International and Consumer Assistance at its toll-free number (800) 638-2041 or (301) 443-6597 or at its Internet addressSincerely yours,
C i P.mVi Lin, Ph.D.
Director
Division of Anesthesiology, General Hospital,
Infection Control and Dental Devices
Office of Device Evaluation
Center for Devices and
Radiological Health
Enclosure
Indications for Use Statement
510(k)
Number
(if known) DeeName 0.9% Sodium Chloride Injection, USP, BD Posiflush SFFlush Syringe
Indications
The0.9% Sodium Chloride Injection, USP, BD Posiflush SF Flush Syringes are intended for use in maintaining for Use patency of vascular access devices (VAD's). PLEASE DO NOT WRITE BELOW THIS LINE -CONTINUE ON ANOTHERPAGE IF NEEDED
Concurrence of CDRH, Office of Device Evaluation (ODE)OR Over-The-Counter Use_
Prescription Use ~
(Per 21 CFR 801. 109) (Division Sign-Off)Division of Anesthesiology, General Hospital,
Infection Control, Dental Devices
510(k) Number:V O 2
000020
quotesdbs_dbs26.pdfusesText_32[PDF] BD Tryptic Soy Broth (TSB)
[PDF] BD-FIL 2014 - Horaires des dédicaces du vendredi 12 septembre - Festival
[PDF] BD-jeunesse_2015-04
[PDF] BD-S1065_FICHE:Mise en page 1
[PDF] Bd. 72 1972 Quellen und Forschungen aus italienischen
[PDF] BDAF120_Decembre2003
[PDF] BDC - Anciens Et Réunions
[PDF] BDC 2014 - Amitié Solidarité Savoie - France
[PDF] bdc 2015 - CE Legrand Lagord - Anciens Et Réunions
[PDF] bdc AGRO - Conception
[PDF] BDC Beaux-Livres réassort - Anciens Et Réunions
[PDF] BDC CIVILITE 2014
[PDF] BDC FDE - Anciens Et Réunions
[PDF] bdc flammes pub - Logiciels Graphiques
