 URGENT MEDICAL DEVICE RECALL
URGENT MEDICAL DEVICE RECALL
16 апр. 2020 г. BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF (Sterile ... Please assist BD with assuring these ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 апр. 2018 г. BD PosiFlush™ Heparin Lock Flush Syringes. 306509 306510
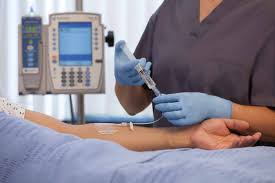 BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
to help reduce complications and improve patient care. BD VAM assessments establish the baseline 5 mL BD PosiFlush™ Heparin Lock Flush Syringe 50 usp units/5 ...
 BD PosiFlushTM Pre-Filled Flush Syringes
BD PosiFlushTM Pre-Filled Flush Syringes
BD PosiFlush™ helps reduce the risk of catheter related bloodstream BD Medical. The Danby Building. Edmund Halley Road. Oxford Science Park. OXFORD OX4 4DQ.
 BD PosiFlush™
BD PosiFlush™
BD Luer-Lok™ Tip Cap: Tighter seal for closure integrity helping prevent touch contamination. Clear labelling helps reduce the risk of medication errors.
 BD PosiFlush™ Prefilled Saline Syringe Supply Update
BD PosiFlush™ Prefilled Saline Syringe Supply Update
Dear BD Valued Customer. We would like to provide you with confidence that BD is working diligently to support your prefilled flush syringe needs through our
 BD Medical Medical Surgical Systems Catalogue Canadian Version
BD Medical Medical Surgical Systems Catalogue Canadian Version
BD Activation-Assist™ technology for fast and easy needle tip shielding more than twice as many BD PosiFlush 3 mL size syringes into a 5.1 L In-Room ...
 Think BD PosiFlush™ Syringes filled with Saline.
Think BD PosiFlush™ Syringes filled with Saline.
BD PosiFlush™ SP Syringes are not intended for use on a sterile field. Page 3. Silicone Lubricant. Medical-grade silicone oil is applied to the stopper and
 TABLE OF CONTENTS
TABLE OF CONTENTS
2 февр. 2017 г. The 0.9% Sodium Chloride Injection USP
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 Apr 2018 bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ HEPARIN LOCK FLUSH ... in Attachment B to aid you with the identification of the.
 BD PosiFlush Recall
BD PosiFlush Recall
16 Apr 2020 BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF. (Sterile Field) Saline Flush Syringe 10mL ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ PRE-FILLED NORMAL SALINE SYRINGE If you require further assistance please contact: Contact.
 URGENT MEDICAL DEVICE RECALL
URGENT MEDICAL DEVICE RECALL
16 Apr 2020 BD is conducting a voluntary medical device recall for multiple lots of the BD PosiFlush™ SF. (Sterile Field) Saline Flush Syringe 10mL ...
 Briefvorlage BD GmbH mit Logo
Briefvorlage BD GmbH mit Logo
20 Apr 2018 bd.com. URGENT MEDICAL DEVICE RECALL. BD POSIFLUSH™ HEPARIN LOCK FLUSH ... in Attachment B to aid you with the identification of the.
 BD PosiFlushTM - Because so much is on the line
BD PosiFlushTM - Because so much is on the line
The BD PosiFlush™ Prefilled Saline Syringe is a solution that is part of the BD Organisation for Standardisation (ISO) 13485 standard on Medical.
 Think BD PosiFlush™ Syringes filled with Saline.
Think BD PosiFlush™ Syringes filled with Saline.
BD PosiFlush™ SP Syringes filled with Saline
 BD Medical – Recall of heparin lock flush and normal saline syringes
BD Medical – Recall of heparin lock flush and normal saline syringes
20 Apr 2018 The BD PosiFlush heparin lock flush syringe is intended to help maintain patency by locking vascular access devices. • The BD pre-filled normal ...
 AUG 2 6 2004 000016
AUG 2 6 2004 000016
6 Feb 2004 USP BD Posiflush SF Pre-Filled Flush Syringe was modified by changing the current ... 1976 the enactment date of the Medical Device.
 BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
BD_Posi-Flush Brochure Option -V8 (I) (with updated CTA no)
to provide clinical benefits to help improve patient care manufacture over 14 billion BD PosiFlush™ Sterile Path Syringes. Saline and syringes.
OptumRx
specializes in the delivery, clinical management and affordability of prescription medications and consumer health products.
We are an Optum
company - a leading provider of integrated health services. Learn more at optum.com.All Optum
trademarks and logos are owned by Optum, Inc. All other brand or product names are trademarks or registered marks of their
respective owners.This document contains information that is considered proprietary to OptumRx and should not be reproduced without the express written
consent of OptumRx.RxNews®
is published by the OptumRx Clinical Services Department.©2018 Optum, Inc. All rights reserved.
optumrx.com BD Medical - Recall of heparin lock flush and normal saline syringes On April 20, 2018, BD Medical announced a recall of some lots of heparin lock flush and normal saline syringes due to a potential for contamination with Serratia marcescens bacterium.Recalled BD
Medical products are listed below:
Product Description Lot #s
BD PosiFlush
Heparin Lock Flush
Syringe Refer to the BD notice for lot
numbers BDPre-Filled Normal Saline
Syringe
The BD PosiFlush heparin lock flush syringe is intended to help maintain patency by locking vascular access devices. The BD pre-filled normal saline syringe is intended for the flushing of indwelling vascular access devices. BD Medical was notified by the FDA and Centers for Disease Control and Prevention (CDC) about a potential epidemiological link between catheter related blood stream infections and the S. marcescens bacterium. The FDA and CDC identified a potential connection between reports of infection in a small number of patients caused by S. marcescens across multiple states. The CDC's initial investigation found that affected patients had received treatment using certain BD flush products. Investigations by BDMedical, the FDA and CDC are currently ongoing.
Anyone with existing inventory of the recalled product should immediately quarantine and discontinue distribution of the product. Patients should contact their healthcare provider if they have experienced any problems that may be related to using the recalled product. Contact BD Medical at 1-866-660-8973 for further questions regarding this recall.quotesdbs_dbs26.pdfusesText_32[PDF] BD Tryptic Soy Broth (TSB)
[PDF] BD-FIL 2014 - Horaires des dédicaces du vendredi 12 septembre - Festival
[PDF] BD-jeunesse_2015-04
[PDF] BD-S1065_FICHE:Mise en page 1
[PDF] Bd. 72 1972 Quellen und Forschungen aus italienischen
[PDF] BDAF120_Decembre2003
[PDF] BDC - Anciens Et Réunions
[PDF] BDC 2014 - Amitié Solidarité Savoie - France
[PDF] bdc 2015 - CE Legrand Lagord - Anciens Et Réunions
[PDF] bdc AGRO - Conception
[PDF] BDC Beaux-Livres réassort - Anciens Et Réunions
[PDF] BDC CIVILITE 2014
[PDF] BDC FDE - Anciens Et Réunions
[PDF] bdc flammes pub - Logiciels Graphiques
