 INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
Unit Written By. Unit No. 1. Dr. K. S. Dhami (Ret. Proff.) 01 02
 Modern inorganic chemistry
Modern inorganic chemistry
Page 1. Modern inorganic chemistry. AN INTERMEDIATE TEXT. C. CHAMBERS B.Sc in first-year tertiary level chemistry courses. The new syllabuses have made it ...
 B. Sc. II YEAR INORGANIC CHEMISTRY-II
B. Sc. II YEAR INORGANIC CHEMISTRY-II
INORGANIC CHEMISTRY-II. BSCCH-201. UTTARAKHAND OPEN UNIVERSITY. Page 1. UNIT 1: CHEMISTRY OF THE ELEMENTS OF. FIRST TRANSITION (3-d) SERIES. CONTENTS: 1.1
 STATE MODEL SYLLABUS FOR UNDER GRADUATE COURSE IN
STATE MODEL SYLLABUS FOR UNDER GRADUATE COURSE IN
Chand Publisher. 2012. Reference Books: 1. Y R Sharma
 Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Department of Chemistry. Inorganic Chemistry (paper code - CH-201). B.Sc 1st Year (Sem. 2nd). GDC Memorial College. (Approved by Govt. of Haryana & Affiliated
 1 INDIRA GANDHI UNIVERSITY MEERPUR (REWARI)
1 INDIRA GANDHI UNIVERSITY MEERPUR (REWARI)
B. Sc IIIrd Semester. Paper IX (Theory) Inorganic Chemistry. Max. Marks: 29. CH-301. Time: 3 Hrs
 INORGANIC CHEMISTRY
INORGANIC CHEMISTRY
B.Sc. B.Sc. (Hons.) & M.Sc. Students of Indian Universities as also for Aqueous chemistry of copper (1) (680). Aqueous chemistry of Cu (II) (681).
 Kurukshetra University Kurukshetra Scheme and Syllabi for B.Sc
Kurukshetra University Kurukshetra Scheme and Syllabi for B.Sc
II Year (IIIrd Semester). Paper-VIII (CH-201) Inorganic Chemistry (Theory). M.Marks: 32. Time: 3 Hrs. Note: Nine questions will be set. Q.No.1 based on whole
 Concise Inorganic Chemistry (4th Edition)
Concise Inorganic Chemistry (4th Edition)
Aparl from ant fair dealing' for the purposes of resean:h 1.>r private study or criticism or review. as permitted under the UK Copyright Designs and. Patents
 B.Sc. III YEAR INORGANIC CHEMISTRY-III
B.Sc. III YEAR INORGANIC CHEMISTRY-III
As a mixture of biochemistry and inorganic chemistry bioinorganic chemistry is 8.3.1 Classification of inorganic polymers. 8.3.1 General properties of ...
 INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
INORGANIC CHEMISTRY-I. BSCCH-101. UTTARAKHAND OPEN UNIVERSITY. Page 1 (v) By the year 1900 some 30 more elements had been added to the list of elements ...
 Modern inorganic chemistry
Modern inorganic chemistry
in first-year tertiary level chemistry courses. the facts of inorganic chemistry and in this book the first four chap- ... (Liverpool B.Sc.
 B. SC. CHEMISTRY (Subsidiary)
B. SC. CHEMISTRY (Subsidiary)
B.Sc. COURSE PLAN (Subsidiary). Inorganic Chemistry. 1st Year. 1st Semester : Paper IS & Paper IIS (Group C each). Marks : 16 + 16.
 Notes of inorganic chemistry for BSc II
Notes of inorganic chemistry for BSc II
(1). T on the basis of this concept have been defined in torms of aqueous solutions. This defination is applicable for hydrogen and hydroxyl Compounds.
 B. Sc. II YEAR INORGANIC CHEMISTRY-II
B. Sc. II YEAR INORGANIC CHEMISTRY-II
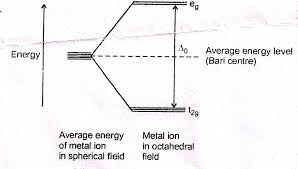
Unit-1 Chemistry of elements of first transition series INORGANIC CHEMISTRY-II. BSCCH-201. UTTARAKHAND OPEN UNIVERSITY. Page 1 ... ion_compounds.pdf.
 Syllabus B.Sc.-Chemistry
Syllabus B.Sc.-Chemistry
[1]. V. B. S. Purvanchal University Jaunpur. Syllabus. B.Sc.-Chemistry. B.Sc.-1- 1. Inorganic Chemistry. Theoretical. 50. 3.00. 2. Organic Chemistry.
 MODEL PAPER FIRST YEAR B.Sc. DEGREE EXAMINATION
MODEL PAPER FIRST YEAR B.Sc. DEGREE EXAMINATION
MODEL PAPER. FIRST YEAR B.Sc. DEGREE EXAMINATION. SEMESTER-I. CHEMISTRY Course-I: INORGANIC & PHYSICAL CHEMISTRY. Time: 3 hours. Maximum Marks: 75.
 t- * rr1*
$---
t- * rr1*
$---
B. Sc. Ist Year (Ist Semester) paper I (Theory) Inorganic chemistry (cH-101) Max. Marks: 27. Time: 3 Hrs. N o t e: The examiners will set seven questions in
 Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Inorganic Chemistry (paper code - CH-101). B.Sc 1st Year (Sem. 1st). GDC Memorial College. (Approved by Govt. of Haryana & Affiliated to M D University
B. SC. CHEMISTRY (Subsidiary)
1st Year 1st Semester
PAPER UNIT PERIODS/WEEK MARKS
IS PC/S-101, OC/S-101, IC/S-101 50
2+2+2IIS PC/S-102, OC/S-102, IC/S-102 50
Chemistry Laboratory : IC/S-Lab. I 6
1st Year 2nd Semester
PAPER UNIT PERIODS/WEEK MARKS
IIIS PC/S-103, OC/S-103, IC/S-103 50
2+2+2IVS PC/S-104, OC/S-104, IC/S-104 50
Chemistry Laboratory : IC/S-Lab. II 6 50+50 [For two semesters]2nd Year 1st Semester
PAPER UNIT PERIODS/WEEK MARKS
VS PC/S-201, OC/S-201, IC/S-201 50
2+2+2VIS PC/S-202, OC/S-202, IC/S-202 50
Chemistry Laboratory : IC/S-Lab. III 6
2nd Year 2nd Semester
PAPER UNIT PERIODS/WEEK MARKS
VIIS PC/S-203, OC/S-203, IC/S-203 50
2+2+2VIIS PC/S-204, OC/S-204, IC/S-204 50
Chemistry Laboratory : OC/S-Lab. I 6 50+50 [For two semesters] GRAND TOTAL 600 Note : IC/S = Inorganic Chemistry (Subsidiary), OC/S = Organic Chemistry (Subsidiary),PC/S = Physical Chemistry (Subsidiary).
B.Sc. COURSE PLAN (Subsidiary)
Inorganic Chemistry
1st Year
1st Semester : Paper IS & Paper IIS (Group C each) Marks : 16 + 16
Unit : IC/S 101 Atomic Structure and Periodic Table : 15 L Unit : IC/S 102 Periodic Properties of Atoms : 15 L2nd Semester : Paper IIIS & Paper IVS (Group C each) Marks : 16 + 16
Unit : IC/S 103 Chemical Bonding - I : 15 L Unit : IC/S 104 Inorganic Equilibrium - I : 15 L2nd Year
1st Semester : Paper VS & Paper VIS Marks : 16 + 16
Unit : IC/S 201 A. Chemical Bonding II
B. Atomic Nuclei & Radioactivity : 15 L
Unit : IC/S 202 Comparative Study of Group elements - I (Non-transition elements of Gr. I, II, III & noble gases) : 15 L2nd Semester : Paper VIIS & Paper VIIIS (Group C each) Marks : 16 + 16
Unit : IC/S 203 A. Introduction to Coordination ChemistryB. Introduction to Transition Metals : 15 L
Unit : IC/S 204 Comparative Study of Group elements - II (Non-transition elements of Gr. IV, V, VI & VII) : 15 LINORGANIC CHEMISTRY (Subsidiary)
1st Year 1st Semester
Unit : IC/S 101 15 L
Atomic Structure and Periodic Table
A. Spectra of many electron system, etc. Recapitulation only. B. The de Broglieconcept of matter wave, the p hO equation (simple problems) C. Quantum mechanical model Schrodinger equation in spherical coordinate (r, ) and its solutions for one electron case (only to write down the expressions for the radial, angular parts of s, p orbitals); significance of the n, l, m quantum numbers; probability interpretation of 2 ,s (only drawing); spin motion of electron and the spin quantum number; many electron system and the Pauli exclusion principle, energy level of A. and writing of the electron configuration of atoms. D. Long form of periodic Table in the light of electronic configurations; classification of elements as sp block (the normal elements); dblockquotesdbs_dbs7.pdfusesText_5[PDF] inscription administrative paris 7 paces
[PDF] inscription cnc 2020
[PDF] inscription concours ecricome 2020
[PDF] inscription dakar
[PDF] inscription faculté de médecine paris 7
[PDF] inscription licence psychologie ied paris 8
[PDF] inscription maternelle 4 ans laval
[PDF] insead
[PDF] insert into table sql
[PDF] insert numeric values sql
[PDF] inside listening and speaking pdf
[PDF] inside tokyo itinerary
[PDF] install fontspec package latex
[PDF] install fortigate
