 INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
Unit Written By. Unit No. 1. Dr. K. S. Dhami (Ret. Proff.) 01 02
 Modern inorganic chemistry
Modern inorganic chemistry
Page 1. Modern inorganic chemistry. AN INTERMEDIATE TEXT. C. CHAMBERS B.Sc in first-year tertiary level chemistry courses. The new syllabuses have made it ...
 B. Sc. II YEAR INORGANIC CHEMISTRY-II
B. Sc. II YEAR INORGANIC CHEMISTRY-II
INORGANIC CHEMISTRY-II. BSCCH-201. UTTARAKHAND OPEN UNIVERSITY. Page 1. UNIT 1: CHEMISTRY OF THE ELEMENTS OF. FIRST TRANSITION (3-d) SERIES. CONTENTS: 1.1
 STATE MODEL SYLLABUS FOR UNDER GRADUATE COURSE IN
STATE MODEL SYLLABUS FOR UNDER GRADUATE COURSE IN
Chand Publisher. 2012. Reference Books: 1. Y R Sharma
 Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Department of Chemistry. Inorganic Chemistry (paper code - CH-201). B.Sc 1st Year (Sem. 2nd). GDC Memorial College. (Approved by Govt. of Haryana & Affiliated
 1 INDIRA GANDHI UNIVERSITY MEERPUR (REWARI)
1 INDIRA GANDHI UNIVERSITY MEERPUR (REWARI)
B. Sc IIIrd Semester. Paper IX (Theory) Inorganic Chemistry. Max. Marks: 29. CH-301. Time: 3 Hrs
 INORGANIC CHEMISTRY
INORGANIC CHEMISTRY
B.Sc. B.Sc. (Hons.) & M.Sc. Students of Indian Universities as also for Aqueous chemistry of copper (1) (680). Aqueous chemistry of Cu (II) (681).
 Kurukshetra University Kurukshetra Scheme and Syllabi for B.Sc
Kurukshetra University Kurukshetra Scheme and Syllabi for B.Sc
II Year (IIIrd Semester). Paper-VIII (CH-201) Inorganic Chemistry (Theory). M.Marks: 32. Time: 3 Hrs. Note: Nine questions will be set. Q.No.1 based on whole
 Concise Inorganic Chemistry (4th Edition)
Concise Inorganic Chemistry (4th Edition)
Aparl from ant fair dealing' for the purposes of resean:h 1.>r private study or criticism or review. as permitted under the UK Copyright Designs and. Patents
 B.Sc. III YEAR INORGANIC CHEMISTRY-III
B.Sc. III YEAR INORGANIC CHEMISTRY-III
As a mixture of biochemistry and inorganic chemistry bioinorganic chemistry is 8.3.1 Classification of inorganic polymers. 8.3.1 General properties of ...
 INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
INORGANIC C B. Sc. I YEAR INORGANIC CHEMISTRY CHEMISTRY-I
INORGANIC CHEMISTRY-I. BSCCH-101. UTTARAKHAND OPEN UNIVERSITY. Page 1 (v) By the year 1900 some 30 more elements had been added to the list of elements ...
 Modern inorganic chemistry
Modern inorganic chemistry
in first-year tertiary level chemistry courses. the facts of inorganic chemistry and in this book the first four chap- ... (Liverpool B.Sc.
 B. SC. CHEMISTRY (Subsidiary)
B. SC. CHEMISTRY (Subsidiary)
B.Sc. COURSE PLAN (Subsidiary). Inorganic Chemistry. 1st Year. 1st Semester : Paper IS & Paper IIS (Group C each). Marks : 16 + 16.
 Notes of inorganic chemistry for BSc II
Notes of inorganic chemistry for BSc II
(1). T on the basis of this concept have been defined in torms of aqueous solutions. This defination is applicable for hydrogen and hydroxyl Compounds.
 B. Sc. II YEAR INORGANIC CHEMISTRY-II
B. Sc. II YEAR INORGANIC CHEMISTRY-II
Unit-1 Chemistry of elements of first transition series INORGANIC CHEMISTRY-II. BSCCH-201. UTTARAKHAND OPEN UNIVERSITY. Page 1 ... ion_compounds.pdf.
 Syllabus B.Sc.-Chemistry
Syllabus B.Sc.-Chemistry
[1]. V. B. S. Purvanchal University Jaunpur. Syllabus. B.Sc.-Chemistry. B.Sc.-1- 1. Inorganic Chemistry. Theoretical. 50. 3.00. 2. Organic Chemistry.
 MODEL PAPER FIRST YEAR B.Sc. DEGREE EXAMINATION
MODEL PAPER FIRST YEAR B.Sc. DEGREE EXAMINATION
MODEL PAPER. FIRST YEAR B.Sc. DEGREE EXAMINATION. SEMESTER-I. CHEMISTRY Course-I: INORGANIC & PHYSICAL CHEMISTRY. Time: 3 hours. Maximum Marks: 75.
 t- * rr1*
$---
t- * rr1*
$---
B. Sc. Ist Year (Ist Semester) paper I (Theory) Inorganic chemistry (cH-101) Max. Marks: 27. Time: 3 Hrs. N o t e: The examiners will set seven questions in
 Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Inorganic Chemistry (paper code - CH-101) B.Sc 1st Year (Sem. 1st)
Inorganic Chemistry (paper code - CH-101). B.Sc 1st Year (Sem. 1st). GDC Memorial College. (Approved by Govt. of Haryana & Affiliated to M D University
SCHOOL OF SCIENCES DEPARTMENT OF CHEMISTRY
UTTARAKHAND OPEN UNIVERSITY
II I I I I I I I I I I I INORGAICR IHEMSTY-TBB--2I-TBB-0I
1RUUIKDAAICR IBPHHBPHSH-EI
VagICR IHEMSTY-TS-0-2Ie I:aFUIFGKRL(R( a3 FGI
Od)).ll(R( a3 FGI
%N4H& NA '!&E( CHNAB )N*T& 'T+ODirector, School of Sciences
Uttarakhand Open University
CHNAB %B 'B '4H4(,4!
Professor Chemistry
Department of Chemistry
School of Sciences, IGNOU, New Delhi CHNA 'B CB 'B -EO!4Professor Chemistry
Department of Chemistry
DSB Campus, Kumaun University
Nainital CHNAB .B 'B /4,4!
Professor Chemistry
Department of Chemistry
Delhi University, Delhi .HB 0O4H 0B C4T!
Programme Coordinator
Department of Chemistry
School of Sciences,
Uttarakhand Open University
Haldwani, Nainital CHN+H4E 0NNH&T4!NH(
1T! 2H!!ET %3
1T! SNB fB .HB 4B 'B .O4 5/E!B CHNAAB6 Lf3 L23 LR3 L1 7 LK Department of Chemistry
D.S.B. Campus, Kumaun University
Nainital
2B .HB )EE!4 D,4H Ld3 L83 LY 7 L-
Department of Chemistry
D.S.B. Campus, Kumaun University
Nainital
0NH(E F&!NH CHNAB %B'B '4H4(,4!
Professor of Chemistry (Retd.)
School of Sciences,
Indira Gandhi National Open University (IGNOU),
Maidan Garhi, New Delhi - 110068
C9I(OE& 93 # 1!!4H4:O4T& ;"ET 1T*EH(!33 <4I&,4T3 S4T!4IM 2dRfR- .HB 'O4IT 'T+O (Assistant Professor)Department of Chemistry
School of Sciences,
Uttarakhand Open University
Haldwani, Nainital
D!IE # ='%S SNB #0N"3H+O! #
F&!NT # =TNH+4T 0OE(!H3 == -8YM-RM-LY1KML1ML1!!4H4:O4T& ;"ET 1T*EH(!3
2L2f INORGARGCI
I IHONEMISITMI HONEIAHAYARGCII
HONEM-IBMI HONEIAHAYARGC-
IIII I IHONEMIaINORNAgGCIOeIKNU1CIKR1I KCACI
I 1 INORGANIC CHEMISTRY-II BSCCH-201UTTARAKHAND OPEN UNIVERSITY Page 1
UNIT 1: CHEMISTRY OF THE ELEMENTS OF FIRST TRANSITION (3-d) SERIES CONTENTS:1.1 Objectives
1.2 Introduction
1.3 Characteristic Properties of d-Block Elements
1.4 Properties of the Elements of the First Transition series
1.5 Binary Compounds and Complexes
1.6 Relative Stability of their Oxidation States
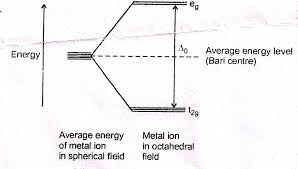
1.7 Coordination number and Geometry
1.8 Summary
1.9 Terminal Questions
1.10 Answers
1.1 OBJECTIVES The objective of writing the text material of this unit is to acquaint the readers to the
characteristic properties of the d-block elements, in general, such as their general electronic configuration and variable oxidation states, complex formation tendency, magnetic properties, formation of coloured ions/compounds, catalytic activity, etc. and periodic properties, viz., atomic radii, atomic volume, ionic radii, melting and boiling points, ionization energies and reactivity, standard electrode potentials and reducing properties, etc. along with their periodic variation along the series. It is also aimed at throwing light on the above properties of the first transition series, in particular, to illustrate the relative stability of the oxidation states of these elements along with to discuss the coordination number and geometry of their complexes and the binary compounds of these elements.1.2 INTRODUCTION The d-block elements have been defined as "the elements whose atoms
receive the last electron in the d-subshell belonging to the penultimate or (n-1)th shell". The d-block elements are also called the transition elements or metals. This is because they exhibit gradual transitional behaviour between highly reactive s-block INORGANIC CHEMISTRY-II BSCCH-201UTTARAKHAND OPEN UNIVERSITY Page 2
(electropositive) and p-block (electronegative) elements, i.e. their properties have been found to be intermediate between those of the s-block and p-block elements. Thus these elements are located in the middle of the periodic table and are the members of the Groups 3 to 12 (IIIB to VIII to II B) in the modern periodic table.quotesdbs_dbs3.pdfusesText_6[PDF] inscription administrative paris 7 paces
[PDF] inscription cnc 2020
[PDF] inscription concours ecricome 2020
[PDF] inscription dakar
[PDF] inscription faculté de médecine paris 7
[PDF] inscription licence psychologie ied paris 8
[PDF] inscription maternelle 4 ans laval
[PDF] insead
[PDF] insert into table sql
[PDF] insert numeric values sql
[PDF] inside listening and speaking pdf
[PDF] inside tokyo itinerary
[PDF] install fontspec package latex
[PDF] install fortigate
